Infrastructure for Asian Connectivity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Pin-Outs (PDF)
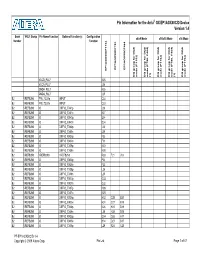
Pin Information for the Arria® GX EP1AGX50C/D Device Version 1.4 Bank VREF Group Pin Name/Function Optional Function(s) Configuration x8/x9 Mode x16/x18 Mode x36 Mode Number Function EP1AGX50DF780 EP1AGX50CF484 EP1AGX50DF1152 DQ group for DQS DQS for group DQ (F1152) mode DQS for group DQ (F780, F484) mode (1) DQS for group DQ (F1152) mode DQS for group DQ (F780, F484) mode (1) DQS for group DQ (F1152) mode VCCD_PLL7 K25 VCCA_PLL7 J26 GNDA_PLL7 K26 GNDA_PLL7 J25 B2 VREFB2N0 FPLL7CLKp INPUT C34 B2 VREFB2N0 FPLL7CLKn INPUT C33 B2 VREFB2N0 IO DIFFIO_TX41p J28 B2 VREFB2N0 IO DIFFIO_TX41n K27 B2 VREFB2N0 IO DIFFIO_RX40p E34 B2 VREFB2N0 IO DIFFIO_RX40n D34 B2 VREFB2N0 IO DIFFIO_TX40p J30 B2 VREFB2N0 IO DIFFIO_TX40n J29 B2 VREFB2N0 IO DIFFIO_RX39p F32 B2 VREFB2N0 IO DIFFIO_RX39n F31 B2 VREFB2N0 IO DIFFIO_TX39p K30 B2 VREFB2N0 IO DIFFIO_TX39n K29 B2 VREFB2N0 VREFB2N0 VREFB2N0 R30 T21 J18 B2 VREFB2N0 IO DIFFIO_RX38p F34 B2 VREFB2N0 IO DIFFIO_RX38n F33 B2 VREFB2N0 IO DIFFIO_TX38p L26 B2 VREFB2N0 IO DIFFIO_TX38n L25 B2 VREFB2N0 IO DIFFIO_RX37p G33 B2 VREFB2N0 IO DIFFIO_RX37n G32 B2 VREFB2N0 IO DIFFIO_TX37p M26 B2 VREFB2N0 IO DIFFIO_TX37n M25 B2 VREFB2N0 IO DIFFIO_RX36p H32 C28 B20 B2 VREFB2N0 IO DIFFIO_RX36n H31 C27 B19 B2 VREFB2N0 IO DIFFIO_TX36p K28 H23 D19 B2 VREFB2N0 IO DIFFIO_TX36n L28 H22 D18 B2 VREFB2N0 IO DIFFIO_RX35p G34 D28 A17 B2 VREFB2N0 IO DIFFIO_RX35n H34 D27 B17 B2 VREFB2N0 IO DIFFIO_TX35p L29 F24 C20 PT-EP1AGX50C/D-1.4 Copyright © 2009 Altera Corp. Pin List Page 1 of 47 Pin Information for the Arria® GX EP1AGX50C/D Device Version -

Max Petroleum Plc Annual Report & Accounts 2013 M a X Petro Le Um P Lc a N N Ua L R E P Ort & a Ccou N Ts 2
Max Petroleum Plc Max Petroleum Plc Annual Report & Accounts 2013 Annual ReportAnnual 2013 & Accounts Having comprehensively restructured its borrowings and extended the exploration period of its Blocks A&E Licence, Max Petroleum is executing an extensive appraisal and development programme to realise the value of its post-salt assets while seeking partners to fund the completion of its potentially transformational NUR-1 well in the pre-salt. Contents 01 Highlights 40 Directors’ Remuneration 02 Joint Chairmen’s Statement Report 04 Company Overview 44 Corporate Governance 08 Key Performance Indicators Report 10 Life Cycle of a Field 46 Independent Auditors’ 11 Production Report 11 Netbacks 47 Financial Statements 12 NUR-1 Well 53 Notes to the Financial 14 Business Review Statements 26 Financial Review 92 Supplemental Disclosure 32 Corporate and Social – Oil and Gas Reserves and Responsibility Resources (unaudited) 34 Board of Directors 93 Glossary 36 Directors’ Report 96 Corporate Directory www.maxpetroleum.com — Revenue of US$93.3 million during the year ended 31 March 2013, up 86% compared to US$50.2 million HIGHLIGHTS during the year ended 31 March 2012. — Average realised selling prices increased 51% as a result of 2013 increased exports relative to domestic sales since the Zhana Makat field entered full field development, providing the Group with the right to export up to 80% of the field’s production. — Entered into a US$90 million loan agreement with SB Sberbank JSC to refinance the Group’s senior debt facility, redeem all of the Group’s convertible bonds for a US$93 million combination of cash and shares, and provide up to US$36.6 million for drilling future post-salt wells. -

Modification of Transition-Metal Redox by Interstitial Water In
Modification of Transition-Metal Redox by Interstitial Water in Hexacyanometallate Electrodes for Sodium-Ion Batteries Jinpeng Wu†, #, Jie Song‡, Kehua Dai※, #, Zengqing Zhuo§, #, L. Andrew Wray⊥, Gao Liu#, Zhi-xun Shen†, Rong Zeng*, ‖, Yuhao Lu*, ‡, Wanli Yang*, # †Geballe Laboratory for Advanced Materials, Stanford University, Stanford, California 94305, USA #Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, California 94720, United States ‡Novasis Energies, Inc., Vancouver, Washington, 98683, United States ※School of Metallurgy, Northeastern University, Shenyang 110819, China §School of Advanced Materials, Peking University Shenzhen Graduate School, Shenzhen 518055, China ⊥Department of Physics, New York University, New York, New York 10003, United States ‖Department of Electrical Engineering, Tsinghua University, Beijing 100084, China ABSTRACT A Sodium-ion battery (SIB) solution is attractive for grid-scale electrical energy storage. Low-cost hexacyanometallate is a promising electrode material for SIBs because of its easy synthesis and open framework. Most hexacyanometallate- based SIBs work with aqueous electrolyte and interstitial water in the material has been found to strongly affect the electrochemical profile, but the mechanism remains elusive. Here we provide a comparative study of the transition-metal redox in hexacyanometallate electrodes with and without interstitial water based on soft X-ray absorption spectroscopy and theoretical calculations. We found distinct transition-metal redox sequences in hydrated and anhydrated NaxMnFe(CN)6·zH2O. The Fe and Mn redox in hydrated electrodes are separated and at different potentials, leading to two voltage plateaus. On the contrary, mixed Fe and Mn redox at the same potential range is found in the anhydrated system. This work reveals for the first time that transition-metal redox in batteries could be strongly affected by interstitial molecules that are seemingly spectators. -

2.3 Nepal Road Network
2.3 Nepal Road Network Overview Primary Roads in Nepal Major Road Construction Projects Distance Matrix Road Security Weighbridges and Axle Load Limits Road Class and Surface Conditions Province 1 Province 2 Bagmati Province Gandaki Province Province 5 Karnali Province Sudurpashchim Province Overview Roads are the predominant mode of transport in Nepal. Road network of Nepal is categorized into the strategic road network (SRN), which comprises of highways and feeder roads, and the local road network (LRN), comprising of district roads and Urban roads. Nepal’s road network consists of about 64,500 km of roads. Of these, about 13,500 km belong to the SRN, the core network of national highways and feeder roads connecting district headquarters. (Picture : Nepal Road Standard 2070) The network density is low, at 14 kms per 100 km2 and 0.9 km per 1,000 people. 60% of the road network is concentrated in the lowland (Terai) areas. A Department of Roads (DoR’s) survey shows that 50% of the population of the hill areas still must walk two hours to reach an SRN road. Two of the 77 district headquarters, namely Humla, and Dolpa are yet to be connected to the SRN. Page 1 (Source: Sector Assessment [Summary]: Road Transport) Primary Roads in Nepal S. Rd. Name of Highway Length Node Feature Remarks N. Ref. (km) No. Start Point End Point 1 H01 Mahendra Highway 1027.67 Mechi Bridge, Jhapa Gadda chowki Border, East to West of Country Border Kanchanpur 2 H02 Tribhuvan Highway 159.66 Tribhuvan Statue, Sirsiya Bridge, Birgunj Connects biggest Customs to Capital Tripureshwor Border 3 H03 Arniko Highway 112.83 Maitighar Junction, KTM Friendship Bridge, Connects Chinese border to Capital Kodari Border 4 H04 Prithvi Highway 173.43 Naubise (TRP) Prithvi Chowk, Pokhara Connects Province 3 to Province 4 5 H05 Narayanghat - Mugling 36.16 Pulchowk, Naryanghat Mugling Naryanghat to Mugling Highway (PRM) 6 H06 Dhulikhel Sindhuli 198 Bhittamod border, Dhulikhel (ARM) 135.94 Km. -

Acipenser Nudiventris Lovetzky, 1828 Ship Sturgeon Esturgeon À Barbillons Frangés Esturión Barba De Flecos
Acipenser nudiventris Lovetzky, 1828 Ship Sturgeon Esturgeon à barbillons frangés Esturión barba de flecos Order: ACIPENSERIFORMES Family: ACIPENSERIDAE SUMMARY The Ship Sturgeon Acipenser nudiventris has always been rare in the Black Sea and the Sea of Azov and their tributaries. There were originally two isolated populations in the northern Caspian Sea, but only the Ural River population is thought to exist today. In Iran (southern Caspian Sea), the species is known to migrate in several rivers. Its former range in the Danube River included Austria and Slovakia. Most range States reported that their wild populations are currently low. Restocking programmes are carried out in Azerbaijan, Iran, and Kazakhstan, but although the species has been in captivity for 60 years, broodstock is currently difficult to obtain. The species is rarely raised in captivity and there is no recorded international trade in products from aquaculture. Of all range States, only Iran, Kazakhstan and the Russian Federation authorise commercial catch of the species. During the past decade, annual catches of A. nudiventris have increased in Iran (from 1.9 tonnes (t) in 1990 to 3.5 t in 2000) and in Kazakhstan (from 12 t in 1990 to 23.8 t in 2000). International trade in 1998 totalled 1,156 kg of A. nudiventris caviar imports comprising: 1,004 kg from Iran, 87 kg from Kazakhstan, and 65 kg from the Russian Federation, 42% of which was declared as pre-Convention stock. No export quotas were established for specimens of A. nudiventris for 1998. Caviar export quotas set by range States increased for Kazakhstan from 1,500 kg for 1999 to 2,000 kg for 2000. -

Policy & Governance Committee
AGENDA BOG Policy & Governance Committee Meeting Date: February 12, 2021 Location: Videoconference Chair: Kamron Graham Vice-Chair: Kate Denning Members: Gabriel Chase, Kate Denning, John Grant, Rob Milesnick, Curtis Peterson, Joe Piucci, David Rosen Staff Liaison: Helen M. Hierschbiel Charge: Develops and monitors the governing rules and policies relating to the structure and organization of the bar; ensures that all bar programs and services comply with organizational mandates and achieve desired outcomes. Identifies and brings emerging issues to the BOG for discussion and action. 2021 PGC Work Plan 1. Wellness Task Force Report. Review report and decide whether to pursue any Exhibit Action 10 recommendations. 2. Evidence-Based Decision-Making Policy. Review Futures Task Force recommendation regarding evidence-based decision-making To Be Posted Action 10 policy and consider whether to adopt the recommended policy. 3. HOD Authority. Discuss whether to pursue changes to limits of HOD authority either Exhibit Action 10 through amendments to HOD Rules or Bar Act. 4. OSB Bylaw Overhaul. Review draft of OSB bylaw overhaul, splitting between policies and Exhibit Discussion 20 bylaws. 5. Bar Sponsorship of Lawyer Referral Services. Review issue presented by Legal Ethics Exhibit Discussion 20 Committee. February 12, 2021 Policy & Governance Committee Agenda Page 2 6. Section Program Review. Review feedback Exhibit Discussion 20 regarding proposed changes to bylaws. 7. Approve minutes of January 8, 2021 meeting. Exhibit Action 1 2021 POLICY & GOVERNANCE WORK PLAN February 12, 2021 draft 2021 AREAS OF TO DO TASKS IN PROCESS (PGC) PGC TASKS DONE IN PROCESS (BOG) BOG TASKS FOCUS 1. Identify information needed 1. -

Development of Strategies to Promote and Facilitate the Implementation of the Asian Highway Design Standards
` DEVELOPMENT OF STRATEGIES TO PROMOTE AND FACILITATE THE IMPLEMENTATION OF THE ASIAN HIGHWAY DESIGN STANDARDS Bangkok DECEMBER 2017 1 The views expressed in this publication are those of the authors and do not necessarily reflect the views of the United Nations Secretariat. The opinions, figures and estimates set forth in this publication are the responsibility of the authors, and should not necessarily be considered as reflecting the views or carrying the endorsement of the United Nations. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of firm names and commercial products does not imply the endorsement of the United Nations. This publication is issued without formal editing. 2 Acknowledgements This document was prepared by Mr. Ishtiaque Ahmed, Economic Affairs Officer, Transport Infrastructure Section, Transport Division as the project manager. Valuable advices were extended by Mr. Pierre Chartier, Section Chief, Transport Infrastructure Section and Mr. Yuwei Li, Director, Transport Division of ESCAP. This study report was prepared with the generous support of the Korean Expressway Corporation (KEC) under the direction of Dr Sung-Min Cho, Director of R&D Planning Office. Fourteen Asian Highway member countries participated in the survey prepared and conducted by the secretariat and provided information on the Asian Highway in their respective territories. Experts and delegates from member countries of the Asian Highway Network have offered valuable comments on the development of this document. -

The Caspian Sea Encyclopedia
Encyclopedia of Seas The Caspian Sea Encyclopedia Bearbeitet von Igor S. Zonn, Aleksey N Kosarev, Michael H. Glantz, Andrey G. Kostianoy 1. Auflage 2010. Buch. xi, 525 S. Hardcover ISBN 978 3 642 11523 3 Format (B x L): 17,8 x 25,4 cm Gewicht: 967 g Weitere Fachgebiete > Geologie, Geographie, Klima, Umwelt > Anthropogeographie > Regionalgeographie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. B Babol – a city located 25 km from the Caspian Sea on the east–west road connecting the coastal provinces of Gilan and Mazandaran. Founded in the sixteenth century, it was once a heavy-duty river port. Since the early nineteenth century, it has been one of the major cities in the province. Ruins of some ancient buildings are found here. Food and cotton ginning factories are also located here. The population is over 283 thou as of 2006. Babol – a river flowing into the Caspian Sea near Babolsar. It originates in the Savadhuk Mountains and is one of the major rivers in Iran. Its watershed is 1,630 km2, its length is 78 km, and its width is about 50–60 m at its mouth down to 100 m upstream. Its average discharge is 16 m3/s. The river receives abundant water from snowmelt and rainfall. -

1St IRF Asia Regional Congress & Exhibition
1st IRF Asia Regional Congress & Exhibition Bali, Indonesia November 17–19 , 2014 For Professionals. By Professionals. "Building the Trans-Asia Highway" Bali’s Mandara toll road Executive Summary International Road Federation Better Roads. Better World. 1 International Road Federation | Washington, D.C. ogether with the Ministry of Public Works Indonesia, we chose the theme “Building the Trans-Asia Highway” to bring new emphasis to a visionary project Tthat traces its roots back to 1959. This Congress brought the region’s stakeholders together to identify new and innovative resources to bridge the current financing gap, while also sharing case studies, best practices and new technologies that can all contribute to making the Trans-Asia Highway a reality. This Congress was a direct result of the IRF’s strategic vision to become the world’s leading industry knowledge platform to help countries everywhere progress towards safer, cleaner, more resilient and better connected transportation systems. The Congress was also a reflection of Indonesia’s rising global stature. Already the largest economy in Southeast Asia, Indonesia aims to be one of world’s leading economies, an achievement that will require the continued development of not just its own transportation network, but also that of its neighbors. Thank you for joining us in Bali for this landmark regional event. H.E. Eng. Abdullah A. Al-Mogbel IRF Chairman Minister of Transport, Kingdom of Saudi Arabia Indonesia Hosts the Region’s Premier Transportation Meeting Indonesia was the proud host to the 1st IRF Asia Regional Congress & Exhibition, a regional gathering of more than 700 transportation professionals from 52 countries — including Ministers, senior national and local government officials, academics, civil society organizations and industry leaders. -

Asian Highway Handbook United Nations
ECONOMIC AND SOCIAL COMMISSION FOR ASIA AND THE PACIFIC ASIAN HIGHWAY HANDBOOK UNITED NATIONS New York, 2003 ST/ESCAP/2303 The Asian Highway Handbook was prepared under the direction of the Transport and Tourism Division of the United Nations Economic and Social Commission for Asia and the Pacific. The team of staff members of the Transport and Tourism Division who prepared the Handbook comprised: Fuyo Jenny Yamamoto, Tetsuo Miyairi, Madan B. Regmi, John R. Moon and Barry Cable. Inputs for the tourism- related parts were provided by an external consultant: Imtiaz Muqbil. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has been issued without formal editing. CONTENTS I. INTRODUCTION TO THE ASIAN HIGHWAY………………. 1 1. Concept of the Asian Highway Network……………………………… 1 2. Identifying the Network………………………………………………. 2 3. Current status of the Asian Highway………………………………….. 3 4. Formalization of the Asian Highway Network……………………….. 7 5. Promotion of the Asian Highway……………………………………... 9 6. A Vision of the Future………………………………………………… 10 II. ASIAN HIGHWAY ROUTES IN MEMBER COUNTRIES…... 16 1. Afghanistan……………………………………………………………. 16 2. Armenia……………………………………………………………….. 19 3. Azerbaijan……………………………………………………………... 21 4. Bangladesh……………………………………………………………. 23 5. Bhutan…………………………………………………………………. 27 6. Cambodia……………………………………………………………… 29 7. China…………………………………………………………………... 32 8. Democratic People’s Republic of Korea……………………………… 36 9. Georgia………………………………………………………………... 38 10. India…………………………………………………………………… 41 11. Indonesia………………………………………………………………. 45 12. Islamic Republic of Iran………………………………………………. 49 13 Japan………………………………………………………………….. -

Acipenser Gueldenstaedtii Brandt, 1833 Russian Sturgeon Ossetra
Acipenser gueldenstaedtii Brandt, 1833 Russian Sturgeon Ossetra Order: ACIPENSERIFORMES Family: ACIPENSERIDAE SUMMARY The Russian Sturgeon Acipenser gueldenstaedtii may live to 48 years weighing around 100 kg. The species matures sexually between 8-16 years and most female spawners in the wild are aged 13-23 years. Spawning occurs every 2-3 years. A. gueldenstaedtii has a wide distribution, occurring in the Caspian Sea, Black Sea and the Sea of Azov, and originally in many of the tributaries of these seas. However the construction of dams on virtually all these rivers has significantly reduced the spawning area. The Ural River is one of the few major spawning rivers that has not been dammed. In addition to loss of habitat, A. gueldenstaedtii has been subject to overfishing and egg-production has been disrupted due to environmental pollution. In the early 1990s, around 30% of the population in the Caspian Sea and almost all the stock in the Sea of Azov originated from re-stocking programmes. However, the restocking of the Volga River by Russian hatcheries decreased from 1991-1995 levels by almost one third during 1996- 1998. In contrast, the number of fingerlings released by Azerbaijan, Bulgaria and Iran has generally increased since 1995. Only Iran monitors the success of their restocking programme. The species is widely bred in captivity to produce meat and fry for both domestic and international trade. There is no record of caviar production in captive breeding facilities. When commercial sturgeon catch in the Caspian peaked in 1997, A. gueldenstaedtii comprised almost 80% of the total catch and still provides a major portion of the global caviar production. -

A Thesis Submitted to the Central European University, Department
A thesis submitted to the Department of Environmental Sciences and Policy of Central European University in part fulfilment of the Degree of Master of Science Changes in the hydrological regime of the Ural River and challenges for transboundary cooperation within the UNECE Water Convention CEU eTD Collection Olga KHON July, 2016 Budapest Notes on copyright and the ownership of intellectual property rights: (1) Copyright in text of this thesis rests with the Author. Copies (by any process) either in full, or of extracts, may be made only in accordance with instructions given by the Author and lodged in the Central European University Library. Details may be obtained from the Librarian. This page must form part of any such copies made. Further copies (by any process) of copies made in accordance with such instructions may not be made without the permission (in writing) of the Author. (2) The ownership of any intellectual property rights which may be described in this thesis is vested in the Central European University, subject to any prior agreement to the contrary, and may not be made available for use by third parties without the written permission of the University, which will prescribe the terms and conditions of any such agreement. (3) For bibliographic and reference purposes this thesis should be referred to as: Khon, O. 2016. Changes in the hydrological regime of the Ural River and challenges for transboundary cooperation within the UNECE Water Convention. Master of Science thesis, Central European University, Budapest. Further information on the conditions under which disclosures and exploitation may take place is available from the Head of the Department of Environmental Sciences and Policy, Central European University.