Can an Off-Nominal Landing by an MMRTG-Powered Spacecraft Induce a Special Region on Mars When No Ice Is Present? Robert F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Vertical Files Anderson Reading Room Center for Southwest Research Zimmerman Library
“A” – biographical Abiquiu, NM GUIDE TO THE GENERAL VERTICAL FILES ANDERSON READING ROOM CENTER FOR SOUTHWEST RESEARCH ZIMMERMAN LIBRARY (See UNM Archives Vertical Files http://rmoa.unm.edu/docviewer.php?docId=nmuunmverticalfiles.xml) FOLDER HEADINGS “A” – biographical Alpha folders contain clippings about various misc. individuals, artists, writers, etc, whose names begin with “A.” Alpha folders exist for most letters of the alphabet. Abbey, Edward – author Abeita, Jim – artist – Navajo Abell, Bertha M. – first Anglo born near Albuquerque Abeyta / Abeita – biographical information of people with this surname Abeyta, Tony – painter - Navajo Abiquiu, NM – General – Catholic – Christ in the Desert Monastery – Dam and Reservoir Abo Pass - history. See also Salinas National Monument Abousleman – biographical information of people with this surname Afghanistan War – NM – See also Iraq War Abousleman – biographical information of people with this surname Abrams, Jonathan – art collector Abreu, Margaret Silva – author: Hispanic, folklore, foods Abruzzo, Ben – balloonist. See also Ballooning, Albuquerque Balloon Fiesta Acequias – ditches (canoas, ground wáter, surface wáter, puming, water rights (See also Land Grants; Rio Grande Valley; Water; and Santa Fe - Acequia Madre) Acequias – Albuquerque, map 2005-2006 – ditch system in city Acequias – Colorado (San Luis) Ackerman, Mae N. – Masonic leader Acoma Pueblo - Sky City. See also Indian gaming. See also Pueblos – General; and Onate, Juan de Acuff, Mark – newspaper editor – NM Independent and -

Martian Crater Morphology
ANALYSIS OF THE DEPTH-DIAMETER RELATIONSHIP OF MARTIAN CRATERS A Capstone Experience Thesis Presented by Jared Howenstine Completion Date: May 2006 Approved By: Professor M. Darby Dyar, Astronomy Professor Christopher Condit, Geology Professor Judith Young, Astronomy Abstract Title: Analysis of the Depth-Diameter Relationship of Martian Craters Author: Jared Howenstine, Astronomy Approved By: Judith Young, Astronomy Approved By: M. Darby Dyar, Astronomy Approved By: Christopher Condit, Geology CE Type: Departmental Honors Project Using a gridded version of maritan topography with the computer program Gridview, this project studied the depth-diameter relationship of martian impact craters. The work encompasses 361 profiles of impacts with diameters larger than 15 kilometers and is a continuation of work that was started at the Lunar and Planetary Institute in Houston, Texas under the guidance of Dr. Walter S. Keifer. Using the most ‘pristine,’ or deepest craters in the data a depth-diameter relationship was determined: d = 0.610D 0.327 , where d is the depth of the crater and D is the diameter of the crater, both in kilometers. This relationship can then be used to estimate the theoretical depth of any impact radius, and therefore can be used to estimate the pristine shape of the crater. With a depth-diameter ratio for a particular crater, the measured depth can then be compared to this theoretical value and an estimate of the amount of material within the crater, or fill, can then be calculated. The data includes 140 named impact craters, 3 basins, and 218 other impacts. The named data encompasses all named impact structures of greater than 100 kilometers in diameter. -

RSL), Described in the 5 August 2011 Science Report “Seasonal Flows on Warm Martian Slopes”
Animated GIFs to illustrate activity of Recurring Slope Lineae (RSL), described in the 5 August 2011 Science report “Seasonal Flows on Warm Martian Slopes”. All of these animated GIF files consist of a series of orthorectified HiRISE images over locations with RSL activity. Orthorectification corrects oblique images to show how the scene would appear from directly overhead. We perform this using HiRISE Digital Terrain Models (DTMs) at a scale of 1 m/posting. The orthorectified images are all at 0.25 m/pixel scale (or 0.5 m/pixel in cases noted below), so features smaller than 1-2 m diameter cannot be correctly rectified; the result is that small-scale features like boulders appear to wag back and forth. Each GIF is timed to dwell 2 seconds on the first and last frames and 1 second on intermediate frames, but network or computer performance may cause this to vary. The legend on each image gives the exact HiRISE image identifier; see http://uahirise.org/{image identified} for more information about each image. The legend also marks the year and seasonal identifier (Ls) for each image. The Mars years begin with the first years of Mars exploration by robot spacecraft. Ls stands for areocentric longitude of the sun, dividing the year into 360 degrees to mark the seasons. Ls = 180 is the beginning of southern spring, Ls = 270 is the beginning of southern summer, and Ls = 360 (or 0) is the beginning of southern autumn. Well-preserved crater on floor of Newton Crater Each of the gifs below are enhanced color sequences from different section of this crater slope, starting with faded RSL in MY29 (except one in which the color data was largely missing), then showing a sequence of RSL forming and fading in MY30. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Santa Fe National Historic Trail: Special History Study
Santa Fe National Historic Trail: Special History Study SANTE FE Special History Study COMERCIANTES, ARRIEROS, Y PEONES: THE HISPANOS AND THE SANTA FE TRADE (Merchants, Muleteers, and Peons) Special History Study Santa Fe National Historic Trail by Susan Calafate Boyle Southwest Cultural Resources Center Professional Papers No. 54 Division of History Southwest Region National Park Service 1994 TABLE OF CONTENTS safe/shs/index.htm Last Updated: 30-Sep-2005 http://www.nps.gov/history/history/online_books/safe/index.htm[7/2/2012 3:03:56 PM] Santa Fe National Historic Trail: Special History Study (Table of Contents) SANTA FE Special History Study TABLE OF CONTENTS COVER LIST OF FIGURES INTRODUCTION CHAPTER I - Isolation and Dependency CHAPTER II - Poverty and Neglect CHAPTER III - Going Down the Royal Road CHAPTER IV - Contraband and the Law CHAPTER V - New Mexican Merchants and Mercantile Capitalism CHAPTER VI - Felipe Chavez CHAPTER VII - Other Leading Merchant Families CONCLUSION RECOMMENDATIONS FOR FURTHER STUDY BIBLIOGRAPHY GLOSSARY APPENDIX I APPENDIX II APPENDIX III APPENDIX IV LIST OF ILLUSTRATIONS Figure 1. "Loading Up." J. Gregg marveled at the dexterity and skill with which hispanos harnessed and adjusted packs of merchandise Figure 2. The Santa Fe Trade: An International Trade Network Figure 3. The Santa Fe Trail: Part of an International Trade Network http://www.nps.gov/history/history/online_books/safe/shst.htm[7/2/2012 3:04:00 PM] Santa Fe National Historic Trail: Special History Study (Table of Contents) Figure 4. Pedro Vial pioneered a route that closely resembled the one Santa Fe Traders would follow in the next century Figure 5. -

I Identification and Characterization of Martian Acid-Sulfate Hydrothermal
Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation of Instrumentation Techniques and Geochemical Processes Through Laboratory Experiments and Terrestrial Analog Studies by Sarah Rose Black B.A., State University of New York at Buffalo, 2004 M.S., State University of New York at Buffalo, 2006 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirement for the degree of Doctor of Philosophy Department of Geological Sciences 2018 i This thesis entitled: Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation of Instrumentation Techniques and Geochemical Processes Through Laboratory Experiments and Terrestrial Analog Studies written by Sarah Rose Black has been approved for the Department of Geological Sciences ______________________________________ Dr. Brian M. Hynek ______________________________________ Dr. Alexis Templeton ______________________________________ Dr. Stephen Mojzsis ______________________________________ Dr. Thomas McCollom ______________________________________ Dr. Raina Gough Date: _________________________ The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above mentioned discipline. ii Black, Sarah Rose (Ph.D., Geological Sciences) Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation -

Appendix I Lunar and Martian Nomenclature
APPENDIX I LUNAR AND MARTIAN NOMENCLATURE LUNAR AND MARTIAN NOMENCLATURE A large number of names of craters and other features on the Moon and Mars, were accepted by the IAU General Assemblies X (Moscow, 1958), XI (Berkeley, 1961), XII (Hamburg, 1964), XIV (Brighton, 1970), and XV (Sydney, 1973). The names were suggested by the appropriate IAU Commissions (16 and 17). In particular the Lunar names accepted at the XIVth and XVth General Assemblies were recommended by the 'Working Group on Lunar Nomenclature' under the Chairmanship of Dr D. H. Menzel. The Martian names were suggested by the 'Working Group on Martian Nomenclature' under the Chairmanship of Dr G. de Vaucouleurs. At the XVth General Assembly a new 'Working Group on Planetary System Nomenclature' was formed (Chairman: Dr P. M. Millman) comprising various Task Groups, one for each particular subject. For further references see: [AU Trans. X, 259-263, 1960; XIB, 236-238, 1962; Xlffi, 203-204, 1966; xnffi, 99-105, 1968; XIVB, 63, 129, 139, 1971; Space Sci. Rev. 12, 136-186, 1971. Because at the recent General Assemblies some small changes, or corrections, were made, the complete list of Lunar and Martian Topographic Features is published here. Table 1 Lunar Craters Abbe 58S,174E Balboa 19N,83W Abbot 6N,55E Baldet 54S, 151W Abel 34S,85E Balmer 20S,70E Abul Wafa 2N,ll7E Banachiewicz 5N,80E Adams 32S,69E Banting 26N,16E Aitken 17S,173E Barbier 248, 158E AI-Biruni 18N,93E Barnard 30S,86E Alden 24S, lllE Barringer 29S,151W Aldrin I.4N,22.1E Bartels 24N,90W Alekhin 68S,131W Becquerei -

Exploring the Water Uptake and Release of Mars-Relevant Salt and Surface Analogs Through Raman Microscopy by Katherine M
Exploring the water uptake and release of Mars-relevant salt and surface analogs through Raman microscopy by Katherine M. Primm B.S. University of Central Arkansas, 2012 M.S. University of Colorado Boulder, 2014 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Chemistry 2018 This dissertation entitled: Exploring the water uptake and release of Mars-relevant salt and surface analogs through Raman microscopy written by Katherine M. Primm has been approved for the Department of Chemistry Professor Margaret A. Tolbert (Principal Advisor) Raina V. Gough, Ph. D. Date: The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above-mentioned discipline. ii Abstract Primm, Katherine M. (Ph.D., Chemistry) Exploring the water uptake and release of Mars-relevant salt and surface analogs through Raman microscopy Dissertation directed by Distinguished Professor Margaret A. Tolbert Pure liquid water is not stable on the surface of Mars because of low pressures and temperatures. The possibility of liquid water seemed more achievable since 2008 when the - Phoenix lander detected ~0.5-1% perchlorate (ClO 4 ) in the Martian soil. Perchlorate and chloride (Cl -) salts are of interest because they readily absorb water vapor and transition into aqueous solutions, a process called deliquescence. This thesis explores the interaction of several Martian surface analogs with water. To study the phase transitions of the Mars-relevant surface analogs, we use a Raman microscope and an environmental cell. -

Solar Wind Charge Exchange X-Ray Emission from Mars. Dimitra Koutroumpa, Ronan Modolo, Gérard Chanteur, Jean-Yves Chaufray, V
Solar wind charge exchange X-ray emission from Mars. Dimitra Koutroumpa, Ronan Modolo, Gérard Chanteur, Jean-Yves Chaufray, V. Kharchenko, Rosine Lallement To cite this version: Dimitra Koutroumpa, Ronan Modolo, Gérard Chanteur, Jean-Yves Chaufray, V. Kharchenko, et al.. Solar wind charge exchange X-ray emission from Mars.: Model and data comparison. Astronomy and Astrophysics - A&A, EDP Sciences, 2012, 545, pp.A153. 10.1051/0004-6361/201219720. hal- 00731069 HAL Id: hal-00731069 https://hal.archives-ouvertes.fr/hal-00731069 Submitted on 2 Sep 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A&A 545, A153 (2012) Astronomy DOI: 10.1051/0004-6361/201219720 & c ESO 2012 Astrophysics Solar wind charge exchange X-ray emission from Mars Model and data comparison D. Koutroumpa1, R. Modolo1, G. Chanteur2, J.-Y., Chaufray1, V. Kharchenko3, and R. Lallement4 1 Université Versailles St-Quentin, CNRS/INSU, LATMOS-IPSL, 11 Bd d’Alembert, 78280 Guyancourt, France e-mail: [email protected] 2 Laboratoire de Physique des Plasmas, École Polytechnique, UPMC, CNRS, Palaiseau, France 3 Physics Department, University of Connecticut, Storrs, Connecticut, USA 4 GEPI, Observatoire de Paris, CNRS, 92195 Meudon, France Received 30 May 2012 / Accepted 17 August 2012 ABSTRACT Aims. -

Executive-Order-No.-422-Re.-Travel-Policies-And-Procedures.Pdf
Republic o-r Palau 0/iice oi die <:Presi"iJent P.O. Box 6051, Palau, PW 96940 Tommy E Remengesau, jr. Tel. (6801 767-2403/2828 !lJ~ Fax. (680)767-2424/1662 Email: rop. [email protected] EXECUTIVE ORDER NO. 422 Revising the Republic ofPalau Travel Policies and Procedures for the Executive Branch ofthe National Government WHEREAS, it is necessary to establish and maintain travel policies and procedures for the Executive Branch of the National Government in a single, accessible, and comprehensive document; and WHEREAS, such travel policies and procedures need to reflect current management practices, desires and terminologies; and WHEREAS, it is thus necessary to periodically update and revise the travel policies and procedures to reflect the changing needs and realities of managing travel funded by the Executive Branch; and WHEREAS, the Executive Branch's comprehensive travel policies and procedures were last revised in February of 2017 and require adjustments to account for circumstances that have changed during this time, including an adjustment of the applicable per diem rates to accurately reflect the present cost of travel; NOW, THEREFORE, by virtue of the authority vested in me as President of the Republic of Palau, and pursuant to the Constitution and laws of the Republic of Palau, I do hereby order that the document entitled "Republic of Palau Travel Policies and Procedures for the Executive Branch of the National Government," as revised on April 2019, be put into effect as of the date of this Executive Order. This Executive Order supersedes all previous orders and directives regarding travel policies and procedures, including specifically Executive Order No. -
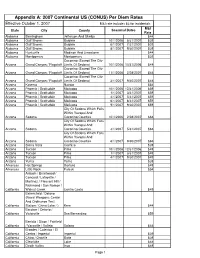
1 Ccr 101-1 State Fiscal Rules 2 (Pdf)
Appendix A: 2007 Continental US (CONUS) Per Diem Rates Effective October 1, 2007 M&I rate includes $3 for incidentals M&I State City County Seasonal Dates Rate Alabama Birmingham Jefferson And Shelby $44 Alabama Gulf Shores Baldwin 10/1/2006 5/31/2007 $39 Alabama Gulf Shores Baldwin 6/1/2007 7/31/2007 $39 Alabama Gulf Shores Baldwin 8/1/2007 9/30/2007 $39 Alabama Huntsville Madison And Limestone $44 Alabama Montgomery Montgomery $39 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 10/1/2006 10/31/2006 $44 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 11/1/2006 2/28/2007 $44 Coconino (Except The City Arizona Grand Canyon / Flagstaff Limits Of Sedona) 3/1/2007 9/30/2007 $44 Arizona Kayenta Navajo $54 Arizona Phoenix / Scottsdale Maricopa 10/1/2006 12/31/2006 $59 Arizona Phoenix / Scottsdale Maricopa 1/1/2007 3/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 4/1/2007 5/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 6/1/2007 8/31/2007 $59 Arizona Phoenix / Scottsdale Maricopa 9/1/2007 9/30/2007 $59 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 10/1/2006 2/28/2007 $64 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 3/1/2007 5/31/2007 $64 City Of Sedona Which Falls Within Yavapai And Arizona Sedona Coconino Counties 6/1/2007 9/30/2007 $64 Arizona Sierra Vista Cochise $39 Arizona Tucson Pima 10/1/2006 12/31/2006 $49 Arizona Tucson Pima 1/1/2007 3/31/2007 $49 Arizona Tucson Pima 4/1/2007 9/30/2007 $49 Arizona Yuma Yuma -

The Prophecy That Is Shaping History
The Prophecy That Is Shaping History: New Research on Ezekiel’s Vision of the End Jon Mark Ruthven, PhD Ihab Griess, PhD Xulon Press 11350 Random Hills Drive #800 Fairfax, Virginia 22030 Copyright Jon Mark Ruthven © 2003 In memoriam Pamela Jessie Ruthven PhD, LCSW 26 March 1952 – 9 April 2001 Wife, mother, and faithful friend i Preface Great events in history often gather momentum and power long before they are recognized by the experts and commentators on world affairs. Easily one of the most neglected but powerfully galvanizing forces shaping history in the world today is the prophecy of Gog and Magog from the 38th and 39th chapters of the book of Ezekiel. This prophecy from the Jewish-Christian Bible has molded geo-politics, not only with- in the United States and the West but also, to an amazing degree, in the Muslim world as well. It seems that, millennia ago, Ezekiel’s vision actually named the nation which millions today believe plays the major role in this prophecy: the nation of Russia. Many modern scholars have dismissed Ezekiel’s Gog and Magog prophecy as a mystical apocalypse written to vindicate the ancient claims of a minor country’s deity. The very notion of such a prediction—that semi-mythical and unrelated nations that dwelt on the fringes of Israel’s geographical consciousness 2,500 years ago would, “in the latter days,” suddenly coalesce into a tidal wave of opposition to a newly regathered state of Jews—seems utterly incredible to a modern mentality. Such a scenario, the experts say, belongs only to the fundamentalist “pop religion” of The Late, Great Planet Earth and of TV evangelists.