University of Copenhagen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2016 IEEE 11Th Conference on Industrial Electronics and Applications (ICIEA 2016)
2016 IEEE 11th Conference on Industrial Electronics and Applications (ICIEA 2016) Hefei, China 5-7 June 2016 Pages 1929-2574 IEEE Catalog Number: CFP1620A-POD ISBN: 978-1-4673-8645-6 4/4 Copyright © 2016 by the Institute of Electrical and Electronics Engineers, Inc All Rights Reserved Copyright and Reprint Permissions: Abstracting is permitted with credit to the source. Libraries are permitted to photocopy beyond the limit of U.S. copyright law for private use of patrons those articles in this volume that carry a code at the bottom of the first page, provided the per-copy fee indicated in the code is paid through Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923. For other copying, reprint or republication permission, write to IEEE Copyrights Manager, IEEE Service Center, 445 Hoes Lane, Piscataway, NJ 08854. All rights reserved. ***This publication is a representation of what appears in the IEEE Digital Libraries. Some format issues inherent in the e-media version may also appear in this print version. IEEE Catalog Number: CFP1620A-POD ISBN (Print-On-Demand): 978-1-4673-8645-6 ISBN (Online): 978-1-4673-8644-9 ISSN: 2156-2318 Additional Copies of This Publication Are Available From: Curran Associates, Inc 57 Morehouse Lane Red Hook, NY 12571 USA Phone: (845) 758-0400 Fax: (845) 758-2633 E-mail: [email protected] Web: www.proceedings.com Technical Programme Session SuA1: Power Electronics (I) Date/Time Sunday, 5 June 2016 / 10:45 – 12:25 Venue 3rd floor Room 1 Chairs Zhenyu Yuan, Northeastern University Maosong Zhang, -

China's Water-Energy-Food R Admap
CHINA’S WATER-ENERGY-FOOD R ADMAP A Global Choke Point Report By Susan Chan Shifflett Jennifer L. Turner Luan Dong Ilaria Mazzocco Bai Yunwen March 2015 Acknowledgments The authors are grateful to the Energy Our CEF research assistants were invaluable Foundation’s China Sustainable Energy in producing this report from editing and fine Program and Skoll Global Threats Fund for tuning by Darius Izad and Xiupei Liang, to their core support to the China Water Energy Siqi Han’s keen eye in creating our infographics. Team exchange and the production of this The chinadialogue team—Alan Wang, Huang Roadmap. This report was also made possible Lushan, Zhao Dongjun—deserves a cheer for thanks to additional funding from the Henry Luce their speedy and superior translation of our report Foundation, Rockefeller Brothers Fund, blue into Chinese. At the last stage we are indebted moon fund, USAID, and Vermont Law School. to Katie Lebling who with a keen eye did the We are also in debt to the participants of the China final copyedits, whipping the text and citations Water-Energy Team who dedicated considerable into shape and CEF research assistant Qinnan time to assist us in the creation of this Roadmap. Zhou who did the final sharpening of the Chinese We also are grateful to those who reviewed the text. Last, but never least, is our graphic designer, near-final version of this publication, in particular, Kathy Butterfield whose creativity in design Vatsal Bhatt, Christine Boyle, Pamela Bush, always makes our text shine. Heather Cooley, Fred Gale, Ed Grumbine, Jia Shaofeng, Jia Yangwen, Peter V. -
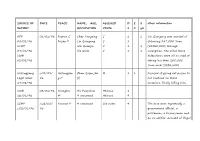
SOURCE of DATE PLACE NAME, AGE, ALLEGED D E 2 Other Information REPORT OCCUPATION CRIME S X Yrs
SOURCE OF DATE PLACE NAME, AGE, ALLEGED D E 2 other information REPORT OCCUPATION CRIME S X yrs AFP 01/01/96 Fuzhou C Chen Yongxing C 1 1 Lin Qiangong was accused of 08/01/96 Fujian P Lin Qiangong C 1 obtaining 247,000 Yuan SCMP Wei Quanjin C 1 1 (US$30,000) through 09/01/96 Xie Qixin C 1 1 corruption. The other three SWB defendants were all accused of 02/02/96 taking less than 200,000 Yuan each (US$2,500) Heilongjiang c.01/01/ Heilongjian Zhan Qiqun,36 M 1 1 Accused of giving rat poison to Legal News 96 g P (f) her husband on three 17/08/96 occasions, finally killing him. SWB 02/01/96 Shanghai Hu Yuanchun Heinous 1 16/01/96 M 9 unnamed Heinous 9 SCMP c.02/01/ Yunnan P 4 unnamed See notes 4 The four were reportedly a c.02/01/96 96 government official, a policeman, a businessman and an ex-soldier. Accused of illegal elephant hunting. SCMP c.02/01/ Shijiazhuan 13 unnamed M, Rob 1 1 c.02/01/96 96 g 3 3 Hebei P FBIS 05/01/96 Shenzhen C 1 unnamed Rob 1 1 Accused of train robbery. 11/01/96 Guangdong SWB P 16/01/96 SWB 05/01/96 Foshan C Lin Zhentao E 1 Accused of embezzling 7.82 02/02/96 Guangdong million Yuan (US$939,759). FBIS P 11/01/96 Shanghai c.08/01/ Shanghai Gao Qiming R 1 Some of these sentences were Legal News 96 M Hu Yuanqing H 1 reportedly suspended for two 08/01/96 Lu Hongbao M 1 years; the report does not SWB Yan Changbing M 1 indicate the names of the 02/02/96 Zhang Xiaodong T 1 prisoners. -

Representing Talented Women in Eighteenth-Century Chinese Painting: Thirteen Female Disciples Seeking Instruction at the Lake Pavilion
REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION By Copyright 2016 Janet C. Chen Submitted to the graduate degree program in Art History and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. ________________________________ Chairperson Marsha Haufler ________________________________ Amy McNair ________________________________ Sherry Fowler ________________________________ Jungsil Jenny Lee ________________________________ Keith McMahon Date Defended: May 13, 2016 The Dissertation Committee for Janet C. Chen certifies that this is the approved version of the following dissertation: REPRESENTING TALENTED WOMEN IN EIGHTEENTH-CENTURY CHINESE PAINTING: THIRTEEN FEMALE DISCIPLES SEEKING INSTRUCTION AT THE LAKE PAVILION ________________________________ Chairperson Marsha Haufler Date approved: May 13, 2016 ii Abstract As the first comprehensive art-historical study of the Qing poet Yuan Mei (1716–97) and the female intellectuals in his circle, this dissertation examines the depictions of these women in an eighteenth-century handscroll, Thirteen Female Disciples Seeking Instructions at the Lake Pavilion, related paintings, and the accompanying inscriptions. Created when an increasing number of women turned to the scholarly arts, in particular painting and poetry, these paintings documented the more receptive attitude of literati toward talented women and their support in the social and artistic lives of female intellectuals. These pictures show the women cultivating themselves through literati activities and poetic meditation in nature or gardens, common tropes in portraits of male scholars. The predominantly male patrons, painters, and colophon authors all took part in the formation of the women’s public identities as poets and artists; the first two determined the visual representations, and the third, through writings, confirmed and elaborated on the designated identities. -

Painters and Publishing in Late Nineteenth-Century Shanghai
Painters and Publishing in Late Nineteenth-century Shanghai JONATHAN HAY The relationship between the art world of Shanghai and the city's pub• lishing industry during the first two decades of the Guangxu reign, from the mid-187os to the mid-r89os, is complex enough to deserve several essays. Here, I shall focus on just one of its aspects: the massive involvement of Shanghai-based painters inproviding designs for illus• trated books closely related to their normal production as painters. These books provide rich material for thematic study, in particular of the late nineteenth-century Chinese representation of metropoli• tan experience; in this essay, ho wever, while I will make a number of such points in passing, I want to defer thematic interpretation in favor of a preliminary survey of the material and a discussion of underlying issues. Since illustrated books of this period have attracted little schol• arly attention, this means classifying the books in question by genre and date and providing information on their contributors and con• tents. One of the implications of taking seriously the mass of printed images is that it then becomes impossible to treat Shanghai painting adequately without taking into account the contributions of those painters to the publishing industry with its very different economic and social relationships. As I shall try to show later, one can legiti• mately use the evidence of the books to explore the larger question of the public space of painting in late nineteenth-century Shanghai, and in the process shed light on the role of illustrated books in China's emergent mass culture. -

List of Publication for Journal of Earth Science in 2020
Journal of Earth Science, Vol. 31, No. 6, p. II–VIII, December 2020 ISSN 1674-487X Printed in China List of Publication for Journal of Earth Science in 2020 No. 1 Paleontology Ufadendron elongatum sp. nov., an Angaran Lycopsid from the Upper Permian of Inner Mongolia, China…………………………1 Zhen Tang, Yi Zhang, Serge V. Naugolnykh, Changqing Zheng, Lu Shi, Tao Qin and Junhao Huang Conodont Biostratigraphy and Age of the Early Triassic Fish-Bearing-Nodule Levels from Nanjing and Jurong, Jiangsu Province, South China…………………………………………………………………………………9 Shuang Liu, Zuoyu Sun, Cheng Ji, Min Zhou and Dayong Jiang Petrology and Geochronology Geochemistry and Geochronology of Diorite in Pengshan Area of Jiangxi Province: Implications for Magmatic Source and Tectonic Evolution of Jiangnan Orogenic Belt………………………………………………23 Hongfeng Shi, Junpeng Wang, Yuan Yao, Jing Zhang, Song Jin, Yingxin Zhu, Kang Jiang, Xiaolong Tian, Deng Xiao and Wenbin Ning Geochronological and Geochemical Constraints on the Petrogenesis of Early Paleoproterozoic (2.40–2.32 Ga) Nb-Enriched Mafic Rocks in Southwestern Yangtze Block and Its Tectonic Implications…………………………………………35 Guichun Liu, Xin Qian, Jing Li, Jian-Wei Zi, Tianyu Zhao, Qinglai Feng, Guangyan Chen and Shaobin Hu Zircon U-Pb Ages and Magmatic History of the Kashan Plutons in the Central Urumieh-Dokhtar Magmatic Arc, Iran: Evidence for Neotethyan Subduction during Paleogene–Neogene………………………………………………………………53 Tayebeh Khaksar, Nematollah Rashidnejad-Omran, Fukun Chen, Shu-Guang Song, Shuang-Qing Li and Majid Ghaderi SIMS -

8 · Chinese Cosmographical Thought: the High Intellectual Tradition
8 · Chinese Cosmographical Thought: The High Intellectual Tradition JOHN B. HENDERSON Chinese cosmographical thought of premodern times was to establish just what these relationships were. Hence this not as concerned as its counterparts in Western civili chapter must rely largely on verbal descriptions for its zations with the overall shape of the world or structure account of Chinese cosmographical thought in its form of the cosmos. There is no pre-seventeenth-century ative phase in the Han era (206 B.C.-A.D. 220), though Chinese equivalent of the medieval European mappae graphic reconstructions dating from the Song and later mundi or of Western representations of the earth show will be included where they are helpful and relevant. ing its various cosmographical divisions or climatic zones. A glance at these reconstructions reveals that most of The widely held conception that China comprised "all them were patterned on the same model, a square divided under heaven" (tianxia), as well as the geographical iso into nine equal squares resembling the form of a simple lation of Chinese civilization, may have contributed to magic square or three-by-three grid. Cosmographers in Chinese cosmographers' lack of interest in outlining, traditional China applied this plan to the conceptuali either realistically or schematically, the form of the world zation and arrangement of such diverse kinds of space as as a whole. Whatever the explanation, traditional Chinese astronomical, political, agrarian, urban, and architectural. cosmographical charts generally represent structures in Just as Sir Thomas Browne, according to Coleridge, saw such microcosmic dimensions as the architectural, the "Quincunxes [or lozenges] in Heaven above, Quincunxes urban, and the agrarian rather than depicting the shape in Earth below, & Quincunxes in the water beneath the of the earth or the system of the world. -

The Mausoleum of Emperor Tang Taizong
SINO-PLATONIC PAPERS Number 187 April, 2009 Zhaoling: The Mausoleum of Emperor Tang Taizong by Xiuqin Zhou Victor H. Mair, Editor Sino-Platonic Papers Department of East Asian Languages and Civilizations University of Pennsylvania Philadelphia, PA 19104-6305 USA [email protected] www.sino-platonic.org SINO-PLATONIC PAPERS is an occasional series edited by Victor H. Mair. The purpose of the series is to make available to specialists and the interested public the results of research that, because of its unconventional or controversial nature, might otherwise go unpublished. The editor actively encourages younger, not yet well established, scholars and independent authors to submit manuscripts for consideration. Contributions in any of the major scholarly languages of the world, including Romanized Modern Standard Mandarin (MSM) and Japanese, are acceptable. In special circumstances, papers written in one of the Sinitic topolects (fangyan) may be considered for publication. Although the chief focus of Sino-Platonic Papers is on the intercultural relations of China with other peoples, challenging and creative studies on a wide variety of philological subjects will be entertained. This series is not the place for safe, sober, and stodgy presentations. Sino-Platonic Papers prefers lively work that, while taking reasonable risks to advance the field, capitalizes on brilliant new insights into the development of civilization. The only style-sheet we honor is that of consistency. Where possible, we prefer the usages of the Journal of Asian Studies. Sinographs (hanzi, also called tetragraphs [fangkuaizi]) and other unusual symbols should be kept to an absolute minimum. Sino-Platonic Papers emphasizes substance over form. -

Commercial Activities and Their Historical Impact in the Three Gorges Area from Seventh to Ninth Century
ISSN 1712-8056[Print] Canadian Social Science ISSN 1923-6697[Online] Vol. 11, No. 6, 2015, pp. 124-127 www.cscanada.net DOI:10.3968/7111 www.cscanada.org Commercial Activities and Their Historical Impact in the Three Gorges Area From Seventh to Ninth Century ZHANG Chaolin[a],* [a]School of History and Culture, Southwest University, Chongqing Tang Dynasty by the Li Family in the early 7th century, the China. country was unified and the political situation was stable. *Corresponding author. The Three Gorges region’s population had continued Received 24 March 2015; accepted 18 May 2015 to grow and get together and the business had become Published online 26 June 2015 increasingly active. Social life and production were more prosperous than any previous dynasty. This paper Abstract attempts to analyze the rise of commercial economy from From Seventh to Ninth century, business in the Three Seventh to Ninth Century in the Three Gorges region and Gorges region along Yangtze River in China had its reasons and to explore the historical impact of this presented unprecedented prosperity which featured phenomenon. the growing business groups, flourishing salt trades, many commodity markets and active shipping trading. 1. THE RISE OF COMMERCIAL The prosperity of the commercial economy in the Three Gorges region during this period of time was attributed ACTIVITIES IN THE THREE GORGES to many factors including the natural conditions, the AREA ALONG YANGTZE RIVER FROM population growth, the development of shipping and TH TH local specialties and others. During this period of time, 7 TO 9 CENTURY the business prosperity in the Three Gorges region had From the Seventh to Ninth Century in the Three Gorges promoted the local economic and social development and region, the commercial activities become unprecedentedly also established its important position of the trade and prosperous, which mainly manifested in the growth of commerce in the upper Yangtze River region. -

2011 2Nd International Conference on Artificial Intelligence, Management Science and Electronic Commerce
2011 2nd International Conference on Artificial Intelligence, Management Science and Electronic Commerce (AIMSEC 2011) Deng Feng, China 8-10 August 2011 Pages 1-827 IEEE Catalog Number: CFP1117P-PRT ISBN: 978-1-4577-0535-9 1/9 Table Of Content "Three Center Three Level" Exploration and Practice of Experimental Teaching System..............................................1 Jun Yang, Yin Dong, Xiaojun Wang, Ga Zhao 0ption Gambling between Manufacturers in Pollution Treatment Technology Investment Decisions under Tradable Emissions Permits and Technical Uncertainty.......................................................................................5 Yi Yong-xi A Bottleneck Resource Identification Method for Completing the Workpiece Based on the Shortest Delay Time..........9 Wen Ding, Li Hou , Aixia Zhang A Combined Generator Based On Two PMLCGs.........................................................................................................14 Guangqiang Zhang A Data-structure Used to Describe Three -Dimensional Geological Bodies Based on Borehole Data.........................17 Chao Ning, Zhonglin Xiang, Yan Wang, Ruihuai Wang A Framework of Chinese Handwriting Learning, Evaluating and Research System Based on Real-time Handwriting Information Collection...........................................................................................................23 Huizhou Zhao A Grey Relevancy Analysis on the Relationship between Energy Consumption and Economic Growth in Henan province.............................................................................................................................................27 -
Efficient Reprogramming of Naıve-Like Induced Pluripotent
Efficient Reprogramming of Naı¨ve-Like Induced Pluripotent Stem Cells from Porcine Adipose-Derived Stem Cells with a Feeder-Independent and Serum-Free System Yu Zhang., Chao Wei., Pengfei Zhang, Xia Li, Tong Liu, Yong Pu, Yunsheng Li, Zubing Cao, Hongguo Cao, Ya Liu, Xiaorong Zhang*, Yunhai Zhang* Anhui Provincial Laboratory for Local Livestock and Poultry Genetic Resource Conservation and Bio-breeding, Department of Animal Science, College of Animal Science and Technology, Anhui Agricultural University, Hefei, Anhui, People’s Republic of China Abstract Induced pluripotent stem cells (iPSCs) are somatic cells reprogrammed by ectopic expression of transcription factors or small molecule treatment, which resemble embryonic stem cells (ESCs). They hold great promise for improving the generation of genetically modified large animals. However, few porcine iPSCs (piPSCs) lines obtained currently can support development of cloned embryos. Here, we generated iPSCs from porcine adipose-derived stem cells (pADSCs), using drug- inducible expression of defined human factors (Oct4, Sox2, c-Myc and Klf4). Reprogramming of iPSCs from pADSCs was more efficient than from fibroblasts, regardless of using feeder-independent or feeder-dependent manners. By addition of Lif-2i medium containing mouse Lif, CHIR99021 and PD0325901 (Lif-2i), naı¨ve-like piPSCs were obtained under feeder- independent and serum-free conditions. These successfully reprogrammed piPSCs were characterized by short cell cycle intervals, alkaline phosphatase (AP) staining, expression of Oct4, Sox2, Nanog, SSEA3 and SSEA4, and normal karyotypes. The resemblance of piPSCs to naı¨ve ESCs was confirmed by their packed dome morphology, growth after single-cell dissociation, Lif-dependency, up-regulation of Stella and Eras, low expression levels of TRA-1-60, TRA-1-81 and MHC I and activation of both X chromosomes. -
Chronology of Shitao's Life
SHITAO APPENDIX ONE Chronology of Shitao's Life References are given here only for information that is not 6r), as the Shunzhi emperor, accompanied by the appoint- presented elsewhere in this book in fuller form (especially ment of Hong Taiji's younger brother, Dorgon (r 6r 2-50), in Chapters 4-6) and accessible through the index. Here, as regent. as throughout this study, years refer to Chinese lunar years. Most of the places mentioned can be found on r644 Map 3. Where an existing artwork contradicts the dates Fall of Beijing to the Shun regime of Li Zicheng, followed given here for the use of specific signatures and seals, this shortly after by their abandonment of Beijing to Qing will generally mean that I am not convinced of the work's forces. Dorgon proclaimed Qing rule over China in the authenticity (though there will inevitably be cases of over- name of the Shunzhi emperor, who shortly after was sight or ignorance as well). With the existence and loca- brought to Beijing. In south China, resistance to the Man- tion in mainland Chinese libraries of rare publications chus crystallized around different claimants to the Ming and manuscripts by no less than thirty-six of his friends throne, whose regimes are collectively known as the and acquaintances newly established, providing a rich Southern Ming. new vein for biographical research, and with new works by Shitao regularly coming to light, this chronology must r645 be considered provisional) Fall of Nanjing to Qing forces. In Guilin in the ninth r642 month, Zhu Hengjia was attacked and defeated by forces of the Southern Ming Longwu emperor, Zhu Yujian, Shitao was born into the family of the Ming princes of under the command of Qu Shisi, and taken to Fuzhou, Jingjiang, under the name of Zhu Ruoji.