Monkeypox Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poxvirus and Rabies Branch (PRB) Fact Sheet
Poxvirus & Rabies Branch (PRB) The epidemiologists, laboratory scientists, and public health professionals in PRB are responsible for surveillance, control, and prevention of illness and death due to poxviruses and rabies. Our Work Laboratory Reference, Diagnostics, & Training PRB’s provides laboratory reference, diagnostic services, and trainings to state and local health departments, the Department of Defense (DOD), international organizations, and the Laboratory Response Network (over 150 labs across the U.S. prepared to respond to bioterrorism). PRB collaborates with the private sector to develop and evaluate novel diagnostic assays, therapeutics, and vaccines for rabies and pox-related viruses. The branch maintains high-containment laboratories (BSL3 &4) to safely conduct public health research. Research & Surveillance PRB conducts research studies on the microbiology, molecular biology, pathogenesis (disease process), ecology, and evolution of Our Pathogens poxviruses and rabies. The branch monitors the incidence rates of PRB’s Poxvirus Team provides subject matter these diseases in the U.S. and internationally. expertise on: Expert Consultation Orthopoxviruses, including PRB provides consultation regarding poxvirus and rabies- Smallpox associated diseases and their diagnoses, prevention, control, and Monkeypox treatments. Assistance is offered to healthcare providers, academic Cowpox institutions, state and local health departments, other government Vaccinia Virus agencies, and the general public. PRB collaborates with non- governmental organizations to develop risk communication Parapoxviruses, including tools for disease prevention. The branch provides training on Orf Virus simple surveillance techniques and trains healthcare workers on monkeypox in the Democratic Republic of the Congo (DRC). PRB Pseudocowpox collaborates with the national government of Haiti on a rabies surveillance and control program which includes health education, enhanced diagnostics, surveillance, and an extensive dog vaccination campaign. -

WHO | World Health Organization
WHO/CDS/CSR/99.1 Report of the meeting of the Ad Hoc Committee on Orthopoxvirus Infections. Geneva, Switzerland, 14-15 January 1999 World Health Organization Department of Communicable Disease Surveillance and Response This document has been downloaded from the WHO/CSR Web site. The original cover pages and lists of participants are not included. See http://www.who.int/emc for more information. © World Health Organization This document is not a formal publication of the World Health Organization (WHO), and all rights are reserved by the Organization. The document may, however, be freely reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale nor for use in conjunction with commercial purposes. The views expressed in documents by named authors are solely the responsibility of those authors. The mention of specific companies or specific manufacturers' products does no imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Contents Introduction 1 Recent monkeypox outbreaks in the Democratic Republic of Congo 1 Review of the report of the 1994 Ad Hoc Committee on Orthopoxvirus Infections 2 Work in WHO Collaborating Centres 3 Analysis and sequencing of variola virus genomes 3 Biosecurity and physical security of WHO collaborating laboratories 4 Smallpox vaccine stocks and production 4 Deliberate release of smallpox virus 4 Survey of WHO Member States latest position on destruction of variola virus 4 Recommendations 5 List of Participants 6 Page i REPORT OF THE MEETING OF THE AD HOC COMMITTEE ON ORTHOPOXVIRUS INFECTIONS Geneva, Switzerland 14-15 January 1999 Introduction Dr Lindsay Martinez, Director, Communicable Disease Surveillance and Response (CSR), welcomed participants and opened the meeting on behalf of the Director-General of WHO, Dr G.H. -

A Tale of Two Viruses: Coinfections of Monkeypox and Varicella Zoster Virus in the Democratic Republic of Congo
Am. J. Trop. Med. Hyg., 104(2), 2021, pp. 604–611 doi:10.4269/ajtmh.20-0589 Copyright © 2021 by The American Society of Tropical Medicine and Hygiene A Tale of Two Viruses: Coinfections of Monkeypox and Varicella Zoster Virus in the Democratic Republic of Congo Christine M. Hughes,1* Lindy Liu,2,3 Whitni B. Davidson,1 Kay W. Radford,4 Kimberly Wilkins,1 Benjamin Monroe,1 Maureen G. Metcalfe,3 Toutou Likafi,5 Robert Shongo Lushima,6 Joelle Kabamba,7 Beatrice Nguete,5 Jean Malekani,8 Elisabeth Pukuta,9 Stomy Karhemere,9 Jean-Jacques Muyembe Tamfum,9 Emile Okitolonda Wemakoy,5 Mary G. Reynolds,1 D. Scott Schmid,4 and Andrea M. McCollum1 1Poxvirus and Rabies Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 2Bacterial Special Pathogens Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 3Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 4Viral Vaccine Preventable Diseases Branch, Division of Viral Diseases, National Center for Immunizations and Respiratory Diseases, U.S. Centers for Disease Control and Prevention, Atlanta, Georgia; 5Kinshasa School of Public Health, Kinshasa, Democratic Republic of Congo; 6Ministry of Health, Kinshasa, Democratic Republic of Congo; 7U.S. Centers for Disease Control and Prevention, Kinshasa, Democratic Republic of Congo; 8Department of Biology, University of Kinshasa, Kinshasa, Democratic Republic of Congo; 9Institut National de Recherche Biomedicale, ´ Kinshasa, Democratic Republic of Congo Abstract. -

Imported Monkeypox, Singapore
DISPATCHES Imported Monkeypox, Singapore Sarah Ee Fang Yong, Oon Tek Ng, Zheng Jie Marc Ho, Tze Minn Mak, Kalisvar Marimuthu, Shawn Vasoo, Tsin Wen Yeo, Yi Kai Ng, Lin Cui, Zannatul Ferdous, Po Ying Chia, Bryan Jun Wei Aw, Charmaine Malenab Manauis, Constance Khia Ki Low, Guanhao Chan, Xinyi Peh, Poh Lian Lim, Li Ping Angela Chow, Monica Chan, Vernon Jian Ming Lee, Raymond Tzer Pin Lin, Mok Kwee Derrick Heng, Yee Sin Leo In May 2019, we investigated monkeypox in a traveler and public health management for this case, together from Nigeria to Singapore. The public health response with lessons learned and implications for control. included rapid identification of contacts, use of quaran- tine, and postexposure smallpox vaccination. No sec- The Case ondary cases were identified. Countries should develop On May 8, 2019, monkeypox was laboratory-con- surveillance systems to detect emerging infectious dis- firmed in a 38-year-old man from Nigeria who had eases globally. traveled to Singapore. The man resided in Delta State, Nigeria, but had attended a wedding in Ebonyi State onkeypox is a zoonosis endemic to West and during April 21–23, where he reported ingestion of MCentral Africa; human cases were first report- barbecued bushmeat that might have been contami- ed in 1970 (1). An outbreak ongoing in Nigeria since nated. He did not handle raw meat and had no expo- 2017 is the largest documented (2). Exported cases in sure to wild animals or their products. He held an ad- the United Kingdom and Israel were reported from ministrative job and reported no contact with rodents travelers infected in Nigeria in 2018 (3,4). -

Monkeypox Virus Emergence in Wild Chimpanzees Reveals Distinct Clinical Outcomes and Viral Diversity
ARTICLES https://doi.org/10.1038/s41564-020-0706-0 Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity Livia V. Patrono1,6, Kamilla Pléh1,2,6, Liran Samuni2,3, Markus Ulrich1, Caroline Röthemeier1, Andreas Sachse1, Silvia Muschter4, Andreas Nitsche4, Emmanuel Couacy-Hymann5, Christophe Boesch2,3, Roman M. Wittig 2,3, Sébastien Calvignac-Spencer1 and Fabian H. Leendertz 1 ✉ Monkeypox is a viral zoonotic disease on the rise across endemic habitats. Despite the growing importance of monkeypox virus, our knowledge on its host spectrum and sylvatic maintenance is limited. Here, we describe the recent repeated emergence of monkeypox virus in a wild, human-habituated western chimpanzee (Pan troglodytes verus, hereafter chimpanzee) population from Taï National Park, Ivory Coast. Through daily monitoring, we show that further to causing its typical exanthematous syn- drome, monkeypox can present itself as a severe respiratory disease without a diffuse rash. By analysing 949 non-invasively collected samples, we identify the circulation of at least two distinct monkeypox virus lineages and document the shedding of infectious particles in faeces and flies, suggesting that they could mediate indirect transmission. We also show that the carnivo- rous component of the Taï chimpanzees’ diet, mainly consisting of the sympatric monkeys they regularly hunt, did not change nor shift towards rodent consumption (the presumed reservoir) before the outbreaks, suggesting that the sudden emergence of monkeypox virus in this population is probably due to changes in the ecology of the virus itself. Using long-term mortality surveillance data from Taï National Park, we provide evidence of little to no prior viral activity over at least two decades. -

Select Biological Agent and Toxin List
Select Biological Agent and Toxin List HHS Non-Overlap Select Agents and Toxins USDA High Consequence Livestock Pathogens - Crimean-Congo haemorrhagic fever virus and Toxins (Non-Overlap agents and toxins) - Coccidioides posadasii - Akabane virus - Ebola viruses - African swine fever virus - Cercopithecine herpesvirus 1 (Herpes B virus) - African horse sickness virus - Lassa fever virus - Avian influenza virus (highly pathogenic) - Marburg virus - Blue tongue virus (Exotic) - Monkeypox virus - Bovine spongiform encephalopathy agent - Rickettsia prowazekii - Camel pox virus - Rickettsia rickettsii - Classical swine fever virus South American haemorrhagic fever viruses - Cowdria ruminantium (Heartwater) - Junin - Foot and mouth disease virus - Machupo - Goat pox virus - Sabia - Lumpy skin disease virus - Flexal - Japanese encephalitis virus - Guanarito - Malignant catarrhal fever virus (Exotic) Tick-borne encephalitis complex (flavi) viruses - Menangle virus - Central European tick-borne encephalitis - Mycoplasma capricolum/M.F38/M. mycoides capri - Far Eastern tick-borne encephalitis - Mycoplasma mycoides mycoides - Russian spring and summer encephalitis - Newcastle disease virus (VVND) - Kyasanur forest disease - Peste Des Petits Ruminants virus - Omsk hemorrhagic fever - Rinderpest virus - Variola major virus (Smallpox virus) - Sheep pox virus - Variola minor virus (Alastrim) - Swine vesicular disease virus - Yersinia pestis - Vesicular stomatitis virus (Exotic) - Abrin - Conotoxins - Diacetoxyscirpenol Plant Pathogens - Ricin - Liberobacter -

Emergence of Monkeypox — West and Central Africa, 1970–2017
Morbidity and Mortality Weekly Report Emergence of Monkeypox — West and Central Africa, 1970–2017 Kara N. Durski, MPH1; Andrea M. McCollum, PhD2; Yoshinori Nakazawa, PhD2; Brett W. Petersen, MD2; Mary G. Reynolds, PhD2; Sylvie Briand, MD, PhD1; Mamoudou Harouna Djingarey, MD3; Victoria Olson, PhD2; Inger K. Damon, MD, PhD2, Asheena Khalakdina, PhD1 The recent apparent increase in human monkeypox cases Monkeypox is a zoonotic orthopoxvirus with a similar dis- across a wide geographic area, the potential for further spread, ease presentation to smallpox in humans, with the additional and the lack of reliable surveillance have raised the level of distinguishing symptom of lymphdenopathy. After an initial concern for this emerging zoonosis. In November 2017, the febrile prodrome, a centrifugally distributed maculopapular World Health Organization (WHO), in collaboration with rash develops, with lesions often present on the palms of CDC, hosted an informal consultation on monkeypox with the hands and soles of the feet. The infection can last up to researchers, global health partners, ministries of health, and 4 weeks, until crusts separate and a fresh layer of skin is formed. orthopoxvirus experts to review and discuss human monkeypox Sequelae include secondary bacterial infections, respiratory in African countries where cases have been recently detected and distress, bronchopneumonia, gastrointestinal involvement, also identify components of surveillance and response that need dehydration, encephalitis, and ocular infections, which can improvement. Endemic human monkeypox has been reported result in permanent corneal scarring. No specific treatment for from more countries in the past decade than during the previ- a monkeypox virus infection currently exists, and patients are ous 40 years. -

Serological Evidence of Multiple Zoonotic Viral Infections Among Wild Rodents in Barbados
pathogens Article Serological Evidence of Multiple Zoonotic Viral Infections among Wild Rodents in Barbados Kirk Osmond Douglas 1,*, Claire Cayol 2 , Kristian Michael Forbes 3, Thelma Alafia Samuels 4, Olli Vapalahti 5, Tarja Sironen 5 and Marquita Gittens-St. Hilaire 6,7 1 Centre for Biosecurity Studies, The University of the West Indies, Cave Hill, St. Michael BB11000, Barbados 2 Department of Wildlife, Fish, and Environmental Studies, Swedish University of Agricultural Sciences, Skogsmarksgränd 17, 901 83 Umeå, Sweden; [email protected] 3 Department of Biological Sciences, University of Arkansas, Fayetteville, AR 72701, USA; [email protected] 4 Epidemiology Research Unit, Caribbean Institute for Health Research (CAIHR), The University of the West Indies, Mona, Kingston 7, Jamaica; alafi[email protected] 5 Department of Virology, Faculty of Medicine, University of Helsinki, Medicum, Haartmaninkatu 3, 0290 Helsinki, Finland; olli.vapalahti@helsinki.fi (O.V.); tarja.sironen@helsinki.fi (T.S.) 6 Faculty of Medical Sciences, The University of the West Indies, Cave Hill, St. Michael BB11000, Barbados; [email protected] 7 Best–dos Santos Public Health Laboratory, Enmore #6, Lower Collymore Rock, St. Michael BB11155, Barbados * Correspondence: [email protected]; Tel.: +246-417-7468 Abstract: Background: Rodents are reservoirs for several zoonotic pathogens that can cause human infectious diseases, including orthohantaviruses, mammarenaviruses and orthopoxviruses. Evidence exists for these viruses circulating among rodents and causing human infections in the Americas, Citation: Douglas, K.O.; Cayol, C.; but much less evidence exists for their presence in wild rodents in the Caribbean. Methods: Here, Forbes, K.M.; Samuels, T.A.; we conducted serological and molecular investigations of wild rodents in Barbados to determine Vapalahti, O.; Sironen, T.; Gittens-St. -

Health Canada Extends Approval of IMVAMUNE to Immunization Against Monkeypox and Orthopox Viruses
Press Release Health Canada Extends Approval of IMVAMUNE to Immunization against Monkeypox and Orthopox Viruses COPENHAGEN, Denmark, November 12, 2020 – Bavarian Nordic A/S (OMX: BAVA, OTC: BVNRY) announced today that Health Canada has expanded the approval of the Company’s non-replicating smallpox vaccine, IMVAMUNE® to include additional indications – specifically, monkeypox and related orthopoxvirus infections and disease in adults 18 years of age and older determined to be at high risk for exposure. The extended approval was granted following assessment of additional clinical and non-clinical data, generated since IMVAMUNE received approval as a smallpox vaccine by Health Canada in 2013. These data include among others a Phase 3 study which demonstrated that, in terms of efficacy, IMVAMUNE was non-inferior to replicating smallpox vaccines, while also confirming its favorable benefit/risk profile. IMVAMUNE is approved in Canada under the Extraordinary Use New Drugs (EUNDS) Pathway. Paul Chaplin, President and CEO of Bavarian Nordic said. “The extended approval of IMVAMUNE in Canada confirms its potential to prevent infections from a wider range of orthopoxviruses, many of which are naturally occurring. While monkeypox is endemic to certain parts of Africa, orthopoxvirus infections may occur from animals to humans all over the world. Although cases are rare, no vaccine has previously been approved for these indications, and we are pleased to receive this extended approval of IMVAMUNE.” Bavarian Nordic has collaborated with the Canadian authorities since 2008 on supplying IMVAMUNE, initially for the Canadian Department of National Defence. Subsequent to the initial Canadian EUNDS approval in 2013, Bavarian Nordic has also collaborated with the Public Health Agency of Canada for stockpiling of the vaccine for the general population. -

Fact Sheet: Basic Information About Monkeypox
MONKEYPOX FACT SHEET Basic Information about Monkeypox Monkeypox: An Emerging Infectious Disease in North America Monkeypox is a rare viral disease that is found mostly in the rainforest countries of central and west Africa. The disease is called “monkeypox” because it was first discovered in laboratory monkeys in 1958. Blood tests of animals in Africa later found evidence of monkeypox infection in various rodent species. The virus that causes monkeypox was recovered from an African squirrel, which may be the natural host. Laboratory studies showed that the virus could also infect rats, mice, and rabbits. In 1970, monkeypox was identified as the cause of a rash illness in humans in remote African locations. In early June 2003, monkeypox was reported among several residents in the United States who became ill after having contact with sick pet prairie dogs. This is the first evidence of community-acquired monkeypox in the United States. Cause of Monkeypox The disease is caused by Monkeypox virus, which belongs to the orthopoxvirus group of viruses. Other orthopoxviruses that can cause infection in humans include variola (smallpox), vaccinia (used in smallpox vaccine), and cowpox viruses. Signs and Symptoms In humans, the signs and symptoms of monkeypox are similar to those of smallpox, but usually milder. Unlike smallpox, monkeypox causes swollen lymph nodes. The incubation period for monkeypox is about 12 days.The illness begins with fever, headache, muscle aches, backache, swollen lymph nodes, a general feeling of discomfort, and exhaustion. Within 1 to 3 days (sometimes longer) after onset of fever, the patient develops a papular rash (i.e., raised bumps), often first on the face but sometimes initially on other parts of the body. -

Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept
pathogens Article Systematic Review of Important Viral Diseases in Africa in Light of the ‘One Health’ Concept Ravendra P. Chauhan 1 , Zelalem G. Dessie 2,3 , Ayman Noreddin 4,5 and Mohamed E. El Zowalaty 4,6,7,* 1 School of Laboratory Medicine and Medical Sciences, College of Health Sciences, University of KwaZulu-Natal, Durban 4001, South Africa; [email protected] 2 School of Mathematics, Statistics and Computer Science, University of KwaZulu-Natal, Durban 4001, South Africa; [email protected] 3 Department of Statistics, College of Science, Bahir Dar University, Bahir Dar 6000, Ethiopia 4 Infectious Diseases and Anti-Infective Therapy Research Group, Sharjah Medical Research Institute and College of Pharmacy, University of Sharjah, Sharjah 27272, UAE; [email protected] 5 Department of Medicine, School of Medicine, University of California, Irvine, CA 92868, USA 6 Zoonosis Science Center, Department of Medical Biochemistry and Microbiology, Uppsala University, SE 75185 Uppsala, Sweden 7 Division of Virology, Department of Infectious Diseases and St. Jude Center of Excellence for Influenza Research and Surveillance (CEIRS), St Jude Children Research Hospital, Memphis, TN 38105, USA * Correspondence: [email protected] Received: 17 February 2020; Accepted: 7 April 2020; Published: 20 April 2020 Abstract: Emerging and re-emerging viral diseases are of great public health concern. The recent emergence of Severe Acute Respiratory Syndrome (SARS) related coronavirus (SARS-CoV-2) in December 2019 in China, which causes COVID-19 disease in humans, and its current spread to several countries, leading to the first pandemic in history to be caused by a coronavirus, highlights the significance of zoonotic viral diseases. -
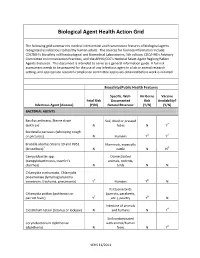
Biological Agent Health Action Grid
Biological Agent Health Action Grid The following grid summarizes medical intervention and transmission features of biological agents recognized as infectious to healthy human adults. The sources for foonote information include: CDC/NIH’s Biosafety in Microbiological and Biomedical Laboratories, 5th edition; CDC/HHS’s Advisory Committee on Immunization Practices; and the APHIS/CDC’s National Select Agent Registry/Select Agents Exclusion. This document is intended to serve as a general information guide. A full risk assessment needs to be prepared for the use of any infectious agent in a lab or animal research setting, and appropriate research compliance committee approvals obtained before work is initiated. Biosafety/Public Health Features Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) BACTERIAL AGENTS Bacillus anthracis, Sterne strain Soil, dried or pressed (anthrax)1 N hides N Y2 Bordetella pertussis (whooping cough or pertussis) N Humans Y3 Y4 Brucella abortus Strains 19 and RB51 Mammals, especially (brucellosis)5 N cattle N N6 Campylobacter spp. Domesticated (campylobacteriosis, traveler's animals, rodents, diarrhea) N birds N N Chlamydia trachomatis, Chlamydia pneumoniae (lymphogranuloma venereum, trachoma, pneumonia) Y7 Humans Y8 N Psittacine birds Chlamydia psittaci (psittacosis or (parrots, parakeets, parrott fever) Y7 etc.), poultry Y8 N Intestine of animals Clostridium tetani (tetanus or lockjaw) N and humans N Y4 Soil contaminated Corynebacterium diphtheriae with animal/human (diphtheria) N feces N Y4 VEHS 11/2011 Specific, Well- Air-Borne Vaccine Fetal Risk Documented Risk Availability? Infectious Agent (disease) (Y/N) Natural Reservoir (Y/N) (Y/N) Francisella tularensis, subspecies Wild animals, esp.