Bioethanol Production by Calcium Alginate-Immobilised St1 Yeast System: Effects of Size of Beads, Ratio and Concentration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Handbook of Jaggery Powder Processing
PM Formalisation of Micro Food Processing Enterprises (PM-FME) Scheme HANDBOOK OF PROCESSING OF JAGGERY AATMANIRBHAR BHARAT Indian Institute of Food Processing Technology Ministry of Food Processing Industries, Government of India Pudukkottai Road, Thanjavur Tamil Nadu Indian Institute of Food Processing Technology TABLE OF CONTENTS Page No. Chapter 1: Introduction 1.1 Status and Market size 2 1.2 Sugarcane products market 5 1.3 Nutritive value of Jaggery 7 1.4 Medicinal properties of jaggery 7 1.5 Jaggery vs Sugar 9 Chapter 2: Processing of Jaggery Powder 2.1 Processing of Jaggery 10 2.2 Traditional production process 11 2.3 Modern scientific method of Jaggery Production 17 2.4 Modified furnace by IISR, Lucknow 21 2.5 Advantages of Modern Technology 23 2.6 Organic jaggery 24 2.7 Jaggery Production: Constraints and remedies Chapter 3: Packaging of Jaggery Powder 3.1 Packaging materials used for Jaggery 26 Chapter 4: Export markets for Jaggery and Jaggery based products 4.1 Export status of jaggery from India 27 4.2 Major constraints identified in jaggery exports suggested by 27 APEDA Chapter 5: Food Safety Regulations and Standards 5.1 Food Safety Regulations & Standards for cane jaggery 29 Machineries Manufacturers & Suppliers 31 Hand book of Processing of Jaggery Page 1 Indian Institute of Food Processing Technology CHAPTER 1 INTRODUCTION 1.1 Status of Sugarcane in India Sugarcane (Saccharum officinarum) family Gramineae (Poaceae) is widely grown crop in India. It provides employment to over a million people directly or indirectly besides contributing significantly to the national exchequer. Sugarcane growing countries of the world lay between the latitude 36.7° north and 31.0° south of the equator extends from tropical to subtropical zones. -

INDIA JONES Grand Platter Korean Set Meal
INDIA JONES Grand Platter (Minimum order for two) 3250 per person APPETISER PLATTER steamed prawn in prik nam pla, chicken satay, chicken and prawn sui mai, fresh Vietnamese rice pa- per rolls with prawn and chicken, Singapore popiah, traditional raw papaya salad Tom Kha Phak vegetable soup with coconut milk or Tom Yam Kai spicy Thai soup with chicken MAIN COURSE PLATTER vegetable green curry, wok fried prawns with seafood sauce, grouper with celery and spring onion, sliced barbecued pork, chicken with chili and ginger, wok fried vegetables in black pepper sauce, Singapore noodles and steamed bread Your choice of sliced fresh fruit or ice cream Korean Set Meal (for lunch only) 2900 DAK DORI TANG stewed farm raised chicken with, leeks, shitake and carrots, essence of ginger, garlic and fresh chilies DO MI JIM steamed red snapper served with sweet soy sauce DEAJI BUL GOGI barbequed pork with chili, sesame seed oil and spring onion All the above main courses will be accompanied with kimchi, namuls, piccata, sticky rice, spinach, cuttle fish and tofu broth and sliced fresh fruits Spicy, (V) Vegetarian preparation, Denotes light and healthy. Should you be allergic to any ingredient please bring it to the attention of the server. Above prices exclude 18% Goods and Services Tax. All our food is cooked in refined vegetable oil or butter. We levy no service charge. 01/08/18 Appetisers EDAMAME (V) : 189 Japan 995 young soy beans lightly salted or with Japanese seven spices CRISP CORN KERNELS (V) China 995 batter fried corn kernels tossed in ‘salt -

Cocinas De Asia Cultura Y Gastronomía
Cocinas de Asia Cultura y Gastronomía Francisco Vintimilla Carrión CEC WCC PCIII 23 / 06 / 2014 Indice 1.1 Introducción a Asia (Ensayo) 1.2 Asia y su importancia en la cocina mundial, productos y herramientas de cocina. 1.3 Religiones de Asia 2.1 China 2.2 China y sus regiones 2.3 Generalidades 2.4 Reseña histórica 2.5 Cultura 2.6 Productos Representativos 2.7 Métodos de cocción 2.8 Cocinas 2.9 Método de servicio y bebidas 2.10 Platos representativos de China 3.1 Japón 3.2 Japón y sus regiones 3.3 Historia 3.4 Generalidades 3.5 Gastronomía 3.6 Métodos de cocción 3.7 Cocinas 3.8 Métodos de servicio y bebidas 3.9 Platos Representativos de Japón 4.1 Corea 4.2 Corea y sus regiones 4.3 Historia 4.4 Cultura y población 4.5 Regiones y Subregiones 4.6 Limites políticos, topografía y clima 4.7 Productos Representativos 4.8 Métodos de cocción 4.9 Cocinas 4.10 Métodos de servicio y bebida 4.11 Platos representativos 5.1 Sureste de Asia 5.2 Sureste de Asia y sus regiones 5.3 Historia 5.4 Cultura y Población 5.5 Regiones y subregiones 5.6 Limites políticos, topografía y clima. 5.7 Productos representativos 5.8 Métodos de cocción. 5.9 Cocinas 5.10 Métodos de servicio y bebidas 5.11 Platos representativos 6.1 India 6.2 India y sus regiones 6.3 Historia 6.4 Cultura y Población 6.5 Regiones y subregiones 6.6 Limites políticos, topografía, clima 6.7 Productos representativos 6.8 Métodos de cocción 6.9 Cocinas 6.10 Métodos de servicio y bebidas 6.11 Platos representativos 7.1 Conclusiones 8.1 Glosario y Fotografías 9.1 Bibliografía 1.1 Introducción (Ensayo “El Arte de la Guerra”). -
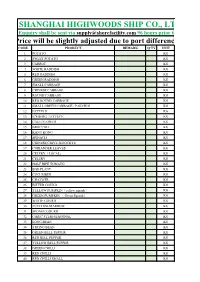
Ship Provisions Loading Application
SHANGHAI HIGHWOODS SHIP CO., LTD. Enquiry shall be sent via [email protected] 96 hours prior to arrival. Price will be slightly adjusted due to port difference CODE. PRODUCT REMARK Q'TY UNIT 1 POTATO KG 2 SWEET POTATO KG 3 CARROT KG 4 WHITE RADDISH KG 5 RED RADDISH KG 6 GREEN RADDISH KG 7 SMALL CABBAGE KG 8 CHINESE CABBAGE KG 9 ROUND CABBAGE KG 10 RED ROUND CABBAGE KG 11 SMALL GREEN CABBAGE / PAKCHOI KG 12 LETTUCE KG 13 ICEBERG LETTUCE KG 14 CAULIFLOWER KG 15 BROCCOLI KG 16 KANG KONG KG 17 SPINACH KG 18 CHINESE CHIVE /KOOCHYE KG 19 CORIANDER LEAVES KG 20 CELERY(LOCAL) KG 21 CELERY KG 22 HALF RIPE TOMATO KG 23 EGG PLANT KG 24 CUCUMBER KG 25 CHAYOTE KG 26 BITTER GOURD KG 27 YELLOW PUMPKIN(yellow squash) KG 28 GREEN PUMPKIN ( Green Squash) KG 29 WHITE GOURD KG 30 ZUCCHINI/MARROW KG 31 SPONGE GOURD KG 32 GARIC STEM-SEASONAL KG 33 LONG BEAN KG 34 STRING BEAN KG 35 GREEN BELL PEPPER KG 36 RED BELL PEPPER KG 37 YELLOW BELL PEPPER KG 38 GREEN CHILLI KG 39 RED CHILLI KG 40 RED CHILLI SMALL KG 41 KOREAN SMALL HOT CHILL KG 42 RED ONION KG 43 WHITE ONION KG 44 LEEK KG 45 SPRING ONION KG 46 GINGER,YOUNG KG 47 GARLIC KG 48 PEELED GARLIC KG 49 GARLIC SPROUTS KG 50 YELLOW BEAN SPROUT KG 51 GREEN BEAN SPROUT KG 52 MUSHROOM KG 53 MUSHROOM,GOLDEN KG 54 OYSTER MUSHROOMS KG 55 SMALL WHITE CIRCLE MUSHROOM KG 56 PLEUROTUS GEESTERANUS KG 57 LOTUS ROOT KG 58 TARO KG 59 LEMON GRASS KG 60 KOREA LETTUCE KG 61 CHRYSANTHEMUM KG 62 FRESH GAI IM/ SESAME LEAF KG 63 FRESH LADY FINGER (OKRA) KG 64 GREEN ASPARAGUS KG 65 BEETROOTS KG 66 KAIK LAN FRESH KG 67 MINT LEAVES -

Methanol Contamination in Indigenous Fermented Alcoholic Beverages
Methanol contamination in indigenous fermented alcoholic beverages Dr. Elijah I. Ohimain Biological Sciences Department Niger Delta University Wilberforce Island, Nigeria Food Safety & Regulatory Measures International conference 17 -19 August 2015 Birmingham, UK Incidences of methanol contamination •Beverage ethanol production via fermentation is an age-long tradition in many parts of the world. •In the tropical world, indigenous/rural people including women are involved in the value chain of traditional alcoholic fermentation • In Africa, Asia and South America, there are increasing incidences of methanol contamination in traditionally fermented alcoholic drinks •For instance, in 2008, more than 180 people were killed in Bangalore and in 2009, 136 people were killed in Gujarat (India) •In 2009, 25 persons died in Indonesia after consuming fermented palm wine containing methanol. •130 Indian villagers die from alcohol poisoning in 2011 •In June 2015, 27 persons died after consuming toxic alcohol in India. •Between April and June 2015, 89 persons died in Nigeria following the consumption of alcohol beverage produced from palm wine WHO (2014) Report • There have been numerous outbreaks of methanol poisoning in recent years in several countries, including Cambodia, Czech Republic, Ecuador, Estonia, India, Indonesia, Kenya, Libya, Nicaragua, Norway, Pakistan, Turkey and Uganda. The size of these outbreaks has ranged from 20 to over 800 victims, with case fatality rates of over 30% in some instances. • Concentrations of 6-27 mg/L have been measured in beer and 10-220 mg/L in spirits. In these concentrations methanol is not harmful. • The informal and illicit production of alcoholic drinks is practiced in many parts of the world, including countries where alcohol is banned. -

LFS Take-Away
Welcome to LFS take-away. Our take-away offerings are very nearly the same as our normal menu minus the hoppers). Orders are for pick-up only and can be ordered via the Bopple app. You will also be able to order some beverages, including a range of our delightful wines. And, what’s more, you may wish to add items from our on-line grocery shop, things in jars and extra food for later… Heat Factor / Vegan / Vegetarian / All gluten free unless indicated BANQUET $70 PP DEVILLED CASHEWS ACHARU PAN ROLL WITH FERMENTED CHILLI SAUCE CURRY VEGETABLE MALLUNG DHAL RICE PAPPADUMS MIXED SAMBOLS MILK TOFFEE CURRIES (CHOOSE ONE) - POTAT0 / CASHEW / FISH / PRAWN / CHICKEN / MEAT SHORT EATS Price TAPIOCA CHIPS $5 A classic Lankan street snack MURUKKU $6 Crispy chickpea batter with crunchy pulses, spices & curry leaves CRAB CUTLETS (4 P/SERVE) (NOT GLUTEN FREE) $16 Deep fried crab balls with a hint of curry powder, green chilli & dill POTATO PAN ROLLS (NOT GLUTEN FREE) With mustard seeds, turmeric & white chillies, served with fermented chilli sauce $8 BEEF PAN ROLLS (NOT GLUTEN FREE) P/PIECE With green chilli, celery & curry powder, served with fermented chilli sauce CURRIES Price POTATO $15 A white curry with turmeric, green chilli & Lankan mustard CASHEW NUT $16 A gentle white curry with cumin seeds FISH $20 White fish with a slightly roasted curry powder PRAWN $22 A fiery red curry soured with tamarind CHICKEN $32 A hot red curry with tomato MEAT $20 A dry black curry with sweet spices VEGETABLES & SIDES Price HERB & ONION SALAD $10 A fresh & healthful -

Traditional Food Recipes
AYUSHMAN BHARAT TRADITIONAL FOOD RECIPES from AYUSH SYSTEMS of MEDICINE Design: Kamleshwar Singh Batra 9810316649 AYUSHMAN BHARAT TRADITIONAL FOOD RECIPES from AYUSH SYSTEMS of MEDICINE Disclaimer: All possible efforts have been made to ensure the correctness of the contents. However, the Ministry of AYUSH shall not be accountable for any inadvertent error in the contents. Corrective measures shall be taken up once such errors are brought to notice. 1 AYUSHMAN BHARAT TRADITIONAL FOOD RECIPES from AYUSH SYSTEMS of MEDICINE Page: Message 4 Foreword 5 Preface 6 Preamble 7 Introduction 8 2 SELECTED FOOD RECIPES Page: 1. Amalaki Panaka (Indian Gooseberry Drink) 11 2. Amla Squash 12 3. Takra (Buttermilk) 13 4. Khalam (Medicated Buttermilk) 14 5. Yusha (Medicated Soup) 15 6. Rasala (Medicated Curd) 16 7. Kharjuradi Mantha (Energy Drink) 17 8. Mamsa Rasam (Medicated Mutton Soup) 18 9. Ragi and Banana Smoothie 19 10. Kulattha Rasam (Horse Gram Rasam) 20 11. Peya (Medicated Rice Gruel) 21 12. Ardraka Paka (Ginger Barfi) 22 13. Madhuka Leha (Herbal Jam) 23 14. Lajardraka (Puffed Paddy Ginger Granules) 24 15. Gulkand (Rose Petal Jam) 25 16. Beetroot Halwa 26 17. Kharjur Laddoo 27 18. Niger Seeds Laddu 28 19. Apoopam (Rice Pancake) 29 20. Pumpkin and Big Beans Sweet Pancake 30 21. Gooseberry Stir Fry 31 22. Patrode (Colocasia Leaf Rolls) 32 23. Mixed Millet Drumstick Leaves Dosa 34 24. Besan-suji Pancake with Sesame Chutney 36 25. Different Varieties of Chutney 38 26. Recipes for Enhancing Lactation 41 Page: Acknowledgement 44 3 AYUSHMAN BHARAT ..................................... TRADITIONAL FOOD RECIPES from AYUSH SYSTEMS of MEDICINE Message Traditional dietary practices that are interweaved with AYUSH systems of medicine place special emphasis on food and diet as a means to good life, health and wellness. -

Health Benefits of Jaggery Tea on the Account of COVID-19
Short Communication iMedPub Journals Health Science Journal 2021 www.imedpub.com Sp. Iss 3: 010 ISSN 1791-809X Health Benefits of Jaggery Tea on the Bhagyashri S Patil* Account of COVID-19 E&TC Department, SMSMPITR, Akluj, Maharashtra, India Abstract *Corresponding author: Consumption of jaggery Tea in winters helps in generating required heat in Bhagyashri S Patil the body, keeps you warm inside. Jaggery has a very good effect on digestion, especially for people who suffer from constipation. It's ideal to have your morning tea made with jaggery as it speeds up digestion after the long hours of fasting. On [email protected] the account of COVID-19 we have found number of health benefits of traditional foods and spices. Multiple numbers of traditional foods are responsible to increase your immunity. Jaggery is one of them. The purpose of this paper is to understand the importance of jaggery. Tel: 09096811271 Keywords: Jaggerytea; Digestion; Body heat E&TC Department, SMSMPITR, Akluj, Maharashtra, India Received with Revision April 21, 2021, Accepted: May 06, 2021, Published: May 10, 2021 Citation: Patil BS (2021) Health Benefits of Introduction Jaggery Tea on the Account of COVID-19. For any tea lover, you would know there is nothing better than Health Sci J. Sp. Iss 3: 010. starting your day with a perfect cup of tea. But due to the sugar in tea, a lot of people have to keep a count of cups they have daily. Though sugar is widely used in many households and tea-houses, it is not so healthy. And for those who like their tea sweet, it can lead to a spike in blood sugar levels. -

FRUITS, BENEFITS, PROCESSING, PRESERVATION and PINEAPPLE RECIPES FRUITS Fruits Are Nature's Wonderful Medicines Packed with Vi
FRUITS, BENEFITS, PROCESSING, PRESERVATION AND PINEAPPLE RECIPES Joy P. P. & Minu Abraham, Pineapple Research Station (Kerala Agricultural University), Vazhakulam-686 670, Muvattupuzha, Ernakulam, Kerala, India, Tel. & Fax: +914852260832, Email: [email protected] FRUITS Fruits are nature’s wonderful medicines packed with vitamins, minerals, anti-oxidants and many phyto-nutrients without which human body cannot maintain proper health and develop resistance to disease. They also contain pectin, cellulose which stimulates intestinal activities and energy giving substances like oils, fats and proteins. Many fruits have medicinal values. Fruits are a high-moisture, generally acidic food that is relatively easy to process and that offers a variety of flavor, aroma, colour and texture to the diet. Fruits, eaten raw or consumed as fresh juice are an excellent way to retain and balance moisture level in a body. The low level of sodium in fruits plays an important role for people who avail of salt free diet. Fruits are an important source of energy. Eating fruit provides health benefits — people who eat more fruits and vegetables as part of an overall healthy diet are likely to have a reduced risk of some chronic diseases. Fruits provide nutrients vital for health and maintenance of our body. However, their availability is seasonal and they are perishable. Hence, they need to be processed to make juices, squashes, jams, etc and preserved. BENEFITS Health Benefits Eating a diet rich in fruits as part of an overall healthy diet may reduce the risk of heart disease, including heart attack, obesity, type 2 diabetes and stroke and may protect against certain types of cancers. -

The Role of Jaggery in Modern Consumer Food Habit - a Review
International Journal of Advanced Scientific Research and Management, Volume 3 Issue 8, Aug 2018 www.ijasrm.com ISSN 2455-6378 The Role of Jaggery in Modern Consumer Food Habit - A Review P. Alamelu mangai1 and K. Subramaniam2 1 PG and Research Department of Commerce, Sri Vasavi College, Erode- 638 316 2 PG and Research Department of Commerce, Erode Arts and Science College, Erode Abstract country. It is also known as Gud, jaggery, vellam and Sugarcane has mainly three processed products viz., bella. Sugar, jaggery and Khandsari of which sugar is a Jaggery is one of the organic food. Jaggery is strategic commodity because it has used world prepared by concentrating the sugarcane juice and it widely. Sugar is used as an additive in various food is available in the form of solid block and in semi- and beverages consumed daily by the world liquid form. Besides this, the sap collected from community. A great need for sugar as a sweetener, some palm trees such as Palmyra palm, (Borassus but people want a low -calorie sweetener and healthy. flabelliser L.), coconut-palm (Cocos nucifera L.), It is suitable with which said that sugar is a purified wild date palm (Phoenix sylvestris Roxb.) and sago- (refined) sugar cane juice after all the vitamins, palm (Caryota urnes L.) is used for preparation of minerals, proteins, enzymes and other beneficial jaggery (Pattnayak and Misra, 2004) for ease of nutrients discharged. In this health conscious era handling, packing and storage. Jaggery in granular where healthy food is preferred over normal food, form is becoming popular. -

Pop Plates, Soups & Salads Good (Mood)
POP PLATES, SOUPS & SALADS GOOD (MOOD) CARBS SIZZLIN’ & BURST BOWLS SWEETS . BEVERAGES D • • GOOD FOO MO OD OD O FO M O D D O • O G G • O D O O D O M F POP is a feeling. O D O O D An infectious feeling of spontaneity O F O M and happiness. Our kitchens want to O D D O • O spread this feeling through some fresh and G G O • fuss-free food. With our heart in Europe but O D D O inspiration from around the world, the menu M O F O journeys with simple and strong flavour pairings. D O O D O Get scrolling and enjoy something old and F O M O D something new with wholesome and fresh D O • O flavours (and a side of good mood)! G G O • O D D O M O F O D O O D O F O M O D D O • O G G O • O D D O M O F O D O POP PLATES Shareable appetizers to pop in your mouth! VEGGIE POPSTICKERS 445 MUSHROOM PUFF Broccoli & mozarella pasta PILLOWS 495 potstickers, tangy tomato ragout Mushroom duxelle puff pillows, & pepper relish assorted mushrooms & parmesan cream BELGIAN FRIES 325 POPPIN’ PHYLLO CONES •garlic-parmesan Roasted vegetables, mushroom, •garlic-lemon salt caramelized onion & goat cheese •celery & cilantro relish in crunchy phyllo cones - 475 Pulled lamb, pepper jam, goat DING DING cheese in crunchy phyllo cones - 525 CHICKEN PINCHOS 495 Chili & malt vinegar baked chicken morsels, CROCCANTE TACOS tomato salmorejo & tanuki crisps Falafel, melted cheddar, pico, hot mayo & pickles folded in a hard-shell bread croccante - 445 GUNPOWDER PANKO SOLE 545 Fried chicken tenders, melted Spiced fish fingers, gunpowder cheddar, pico, hot mayo & pickles drizzle, mustard, -

Sweet Potato Bhel, Apple, Pomegranate (V) 10 Dashi
SEASONAL A GLASS OF BELLINI POL ROGER 11 BRUT NV POMEGRANATE 15 LUNCH & DINNER NEGRONI 10.5 BAR SNACKS Franca's Chickpea Chips, Fenugreek, Bengali Tomato Chutney (v) 6.5 Prawn Toast Scotch Egg, Banana Ketchup, Pickled Cucumber 7.5 Beetroot & Shankleesh Croquetas (v) 7 Clove Smoked Venison Samosa, Beetroot Chutney 8 SMALL PLATES Sweet Potato Bhel, Apple, Pomegranate (v) 10 Dashi Broccoli, Roasted Sesame Sauce, Tobiko (v) 11.5 Cauliflower Popcorn, Chilli & Chinkiang Vinegar Dipping Sauce (v) 9.5 Chicken Skewers, Peanut Sauce, Papaya Achara 12.5 Seared Scallops, Congee, Szechuan Chilli Oil 15.5 BIG PLATES Scottish Mackerel, Brinjal Pickle, Buttermilk Rasam 20 Kuku Paka, Sukuma Wiki, Saffron Rice 17.5 Caramel Braised Tofu, Confit Garlic Rice (v) 18 Chilli Pickled Cucumbers Pumpkin Khichdee (v) or Tiger Prawn Khichdee 15 / 26 Lemon Rice, Moilee Broth, Coconut Chutney Scrag End Pie 18.5 Lamb Neck, Black Cardamom & Turmeric Infused Mash SIDES Okra Fries, Curry Leaf Mayonnaise (v) 6.5 Green Bean & Cashew Nut Thoran (v) 5 Jikoni Block Print Napkins and Tablecloths LUNCH ~ TUE-FRI 12noon-3pm from Jaipur DINNER ~ TUE-SAT 5.30pm-10.30pm Napkins ~ 4 for £20 Tablecloths ~ £45 to £65 BRUNCH ~ SAT 11am-3pm SUN 11am-4pm A discretionary 12.5% service charge will be added to your bill ~ All prices include VAT Dishes may contain traces of nuts/allergens. Please speak to your server regarding any dietary requirements or allergies. jikonilondon.com ~ @jikonilondon DRINKS MENU HOUSE COCKTAILS Rhubarb, Pink Peppercorn & Rose Bellini 11 Kahwa Martini ~ Black