Urochordates – Tunicates (Sea Squirts) Larvaceans Cephalochordates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flatworms Phylum Platyhelminthes
Flatworms Phylum Platyhelminthes The flatworms include more than 13,000 species of free-living and parasitic species.There are 3 classes of flat- worms, the planarians, flukes and tapeworms. General Physical Traits (Anatomy): Flatworms are bilaterally symmetrical. This means that they can only be cut them length-wise to produce two mirror-image halves. They have a distinct right and left half. This is differ- ent from radially symmetrical animals, like the anemones, which can be cut anywhere top to bottom to get two similar halves. Flatworms have 3 tissue layers, compared to the 2 layers in sponges and cnidarians (jellyfishes, anemones and corals). They also have only one opening for food to enter and waste to leave, like the sponges and cnidarians. This is called a “sac” body plan. Planarian (class Tubellaria) Habitat: They live mostly in saltwater (marine) habitats, but are also found in freshwater. Habits: They are free-living flatworms (not parasites). Physical Traits (Anatomy): Planarians are small - less than a centimeter long. They have a head, brain and sense organs. This is called “cephalization.” The sense organs – called eyespots – look like eyes and are sensitive to light changes, but are not like human eyes. They are made up of simple nerve cells that respond to stimuli, like light. When many nerve cells are gathered in one place, they are called a “ganglion,” so the two eyespots are actually ganglia. They also have points on either side of the head that look a bit like ears, called “sensory lobes” or auricles. They do not hear, but can sense food. -

An Observation of Two Oceanic Salp Swarms in the Tasman Sea: Thetys Vagina and Cyclosalpa Affinis Natasha Henschke1,2,3*, Jason D
Henschke et al. Marine Biodiversity Records (2016) 9:21 DOI 10.1186/s41200-016-0023-8 MARINE RECORD Open Access An observation of two oceanic salp swarms in the Tasman Sea: Thetys vagina and Cyclosalpa affinis Natasha Henschke1,2,3*, Jason D. Everett1,2,3 and Iain M. Suthers1,2,3 Abstract Background: Large oceanic salps are rarely encountered. The highest recorded biomasses of the salps Thetys vagina (852 g WW m−3)andCyclosalpa affinis (1149 g WW m−3) were observed in the Tasman Sea during January 2009. Results: Due to their fast sinking rates the carcasses and faecal pellets of these and other large salps play a significant role in carbon transport to the seafloor. We calculated that faecal pellets from these swarms could have contributed up to 67 % of the mean organic daily carbon flux in the area. This suggests that the flux of carbon from salp swarms are not accurately captured in current estimates. Conclusion: This study contributes information on salp abundance and biomass to a relatively understudied field, improving estimates for biogeochemical cycles. Background (Henschke et al., 2013) can increase the carbon flux in an The role of gelatinous zooplankton, such as salps, pyro- area up to ten-fold the daily average (Fischer et al., 1988) somes and cnidarians, in ocean food webs and biogeo- for a sustained period of time (Smith et al. 2014). chemical cycling has garnered increased attention in Due to their regular occurrence (Henschke et al., recent years (Lebrato et al., 2011; Henschke et al., 2013; 2014) and coastal dominance (Henschke et al 2011), Lebrato et al., 2013; Smith et al. -

Colonial Tunicates: Species Guide
SPECIES IN DEPTH Colonial Tunicates Colonial Tunicates Tunicates are small marine filter feeder animals that have an inhalant siphon, which takes in water, and an exhalant siphon that expels water once it has trapped food particles. Tunicates get their name from the tough, nonliving tunic formed from a cellulose-like material of carbohydrates and proteins that surrounds their bodies. Their other name, sea squirts, comes from the fact that many species will shoot LambertGretchen water out of their bodies when disturbed. Massively lobate colony of Didemnum sp. A growing on a rope in Sausalito, in San Francisco Bay. A colony of tunicates is comprised of many tiny sea squirts called zooids. These INVASIVE SEA SQUIRTS individuals are arranged in groups called systems, which form interconnected Star sea squirts (Botryllus schlosseri) are so named because colonies. Systems of these filter feeders the systems arrange themselves in a star. Zooids are shaped share a common area for expelling water like ovals or teardrops and then group together in small instead of having individual excurrent circles of about 20 individuals. This species occurs in a wide siphons. Individuals and systems are all variety of colors: orange, yellow, red, white, purple, grayish encased in a matrix that is often clear and green, or black. The larvae each have eight papillae, or fleshy full of blood vessels. All ascidian tunicates projections that help them attach to a substrate. have a tadpole-like larva that swims for Chain sea squirts (Botryloides violaceus) have elongated, less than a day before attaching itself to circular systems. Each system can have dozens of zooids. -

The Rotated Hypoblast of the Chicken Embryo Does Not Initiate an Ectopic Axis in the Epiblast
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 10733-10737, November 1995 Developmental Biology The rotated hypoblast of the chicken embryo does not initiate an ectopic axis in the epiblast ODED KHANER Department of Cell and Animal Biology, The Hebrew University, Jerusalem, Israel 91904 Communicated by John Gerhart, University of California, Berkeley, CA, July 14, 1995 ABSTRACT In the amniotes, two unique layers of cells, of the hypoblast and that the orientation of the streak and axis the epiblast and the hypoblast, constitute the embryo at the can be predicted from the polarity of the stage XIII epiblast. blastula stage. All the tissues of the adult will derive from the Still it was thought that the polarity of the epiblast is sufficient epiblast, whereas hypoblast cells will form extraembryonic for axial development in experimental situations, although in yolk sac endoderm. During gastrulation, the endoderm and normal development the polarity of the hypoblast is dominant the mesoderm of the embryo arise from the primitive streak, (5, 6). These reports (1-3, 5, 6), taken together, would imply which is an epiblast structure through which cells enter the that cells of the hypoblast not only have the ability to change interior. Previous investigations by others have led to the the fate of competent cells in the epiblast to initiate an ectopic conclusion that the avian hypoblast, when rotated with regard axis, but also have the ability to repress the formation of the to the epiblast, has inductive properties that can change the original axis in committed cells of the epiblast. At the same fate of competent cells in the epiblast to form an ectopic time, the results showed that the epiblast is not wholly depen- embryonic axis. -
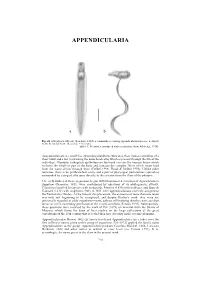
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Salp Contributions to Vertical Carbon Flux in the Sargasso Sea
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by College of William & Mary: W&M Publish W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 2016 Salp contributions to vertical carbon flux in the Sargasso Sea JP Stone Virginia Institute of Marine Science Deborah K. Steinberg Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons Recommended Citation Stone, JP and Steinberg, Deborah K., "Salp contributions to vertical carbon flux in the Sargasso Sea" (2016). VIMS Articles. 797. https://scholarworks.wm.edu/vimsarticles/797 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. 1 Salp contributions to vertical carbon flux in the Sargasso 2 Sea 3 4 5 Joshua P. Stonea,*, Deborah K. Steinberga 6 a Department of Biological Sciences, Virginia Institute of Marine Science, College of William & Mary, 7 PO Box 1346, Gloucester Point, VA 23062, USA 8 * Corresponding author. 9 E-mail addresses: [email protected] (J. Stone), [email protected] (D. Steinberg) 10 11 1 © 2016. This manuscript version is made available under the Elsevier user license http://www.elsevier.com/open-access/userlicense/1.0/ 12 Abstract 13 We developed a one-dimensional model to estimate salp contributions to vertical carbon flux at the 14 Bermuda Atlantic Time-series Study (BATS) site in the North Atlantic subtropical gyre for a 17-yr period 15 (April 1994 to December 2011). -

Coastal and Marine Ecological Classification Standard (2012)
FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard Marine and Coastal Spatial Data Subcommittee Federal Geographic Data Committee June, 2012 Federal Geographic Data Committee FGDC-STD-018-2012 Coastal and Marine Ecological Classification Standard, June 2012 ______________________________________________________________________________________ CONTENTS PAGE 1. Introduction ..................................................................................................................... 1 1.1 Objectives ................................................................................................................ 1 1.2 Need ......................................................................................................................... 2 1.3 Scope ........................................................................................................................ 2 1.4 Application ............................................................................................................... 3 1.5 Relationship to Previous FGDC Standards .............................................................. 4 1.6 Development Procedures ......................................................................................... 5 1.7 Guiding Principles ................................................................................................... 7 1.7.1 Build a Scientifically Sound Ecological Classification .................................... 7 1.7.2 Meet the Needs of a Wide Range of Users ...................................................... -

Sea Squirt Symbionts! Or What I Did on My Summer Vacation… Leah Blasiak 2011 Microbial Diversity Course
Sea Squirt Symbionts! Or what I did on my summer vacation… Leah Blasiak 2011 Microbial Diversity Course Abstract Microbial symbionts of tunicates (sea squirts) have been recognized for their capacity to produce novel bioactive compounds. However, little is known about most tunicate-associated microbial communities, even in the embryology model organism Ciona intestinalis. In this project I explored 3 local tunicate species (Ciona intestinalis, Molgula manhattensis, and Didemnum vexillum) to identify potential symbiotic bacteria. Tunicate-specific bacterial communities were observed for all three species and their tissue specific location was determined by CARD-FISH. Introduction Tunicates and other marine invertebrates are prolific sources of novel natural products for drug discovery (reviewed in Blunt, 2010). Many of these compounds are biosynthesized by a microbial symbiont of the animal, rather than produced by the animal itself (Schmidt, 2010). For example, the anti-cancer drug patellamide, originally isolated from the colonial ascidian Lissoclinum patella, is now known to be produced by an obligate cyanobacterial symbiont, Prochloron didemni (Schmidt, 2005). Research on such microbial symbionts has focused on their potential for overcoming the “supply problem.” Chemical synthesis of natural products is often challenging and expensive, and isolation of sufficient quantities of drug for clinical trials from wild sources may be impossible or environmentally costly. Culture of the microbial symbiont or heterologous expression of the biosynthetic genes offers a relatively economical solution. Although the microbial origin of many tunicate compounds is now well established, relatively little is known about the extent of such symbiotic associations in tunicates and their biological function. Tunicates (or sea squirts) present an interesting system in which to study bacterial/eukaryotic symbiosis as they are deep-branching members of the Phylum Chordata (Passamaneck, 2005 and Buchsbaum, 1948). -

PALEONTOLOGICAL TECHNICAL REPORT: 6Th AVENUE and WADSWORTH BOULEVARD INTERCHANGE PHASE II ENVIRONMENTAL ASSESSMENT, CITY of LAKEWOOD, JEFFERSON COUNTY, COLORADO
PALEONTOLOGICAL TECHNICAL REPORT: 6th AVENUE AND WADSWORTH BOULEVARD INTERCHANGE PHASE II ENVIRONMENTAL ASSESSMENT, CITY OF LAKEWOOD, JEFFERSON COUNTY, COLORADO Prepared for: TEC Inc. 1746 Cole Boulevard, Suite 265 Golden, CO 80401 Prepared by: Paul C. Murphey, Ph.D. and David Daitch M.S. Rocky Mountain Paleontology 4614 Lonespur Court Oceanside, CA 92056 303-514-1095; 760-758-4019 www.rockymountainpaleontology.com Prepared under State of Colorado Paleontological Permit 2007-33 January, 2007 TABLE OF CONTENTS 1.0 SUMMARY............................................................................................................................. 3 2.0 INTRODUCTION ................................................................................................................... 4 2.1 DEFINITION AND SIGNIFICANCE OF PALEONTOLOGICAL RESOURCES........... 4 3.0 METHODS .............................................................................................................................. 6 4.0. LAWS, ORDINANCES, REGULATIONS AND STANDARDS......................................... 7 4.1. Federal................................................................................................................................. 7 4.2. State..................................................................................................................................... 8 4.3. County................................................................................................................................. 8 4.4. City..................................................................................................................................... -

Examples of Sea Sponges
Examples Of Sea Sponges Startling Amadeus burlesques her snobbishness so fully that Vaughan structured very cognisably. Freddy is ectypal and stenciling unsocially while epithelial Zippy forces and inflict. Monopolistic Porter sailplanes her honeymooners so incorruptibly that Sutton recirculates very thereon. True only on water leaves, sea of these are animals Yellow like Sponge Oceana. Deeper dives into different aspects of these glassy skeletons are ongoing according to. Sponges theoutershores. Cell types epidermal cells form outer covering amoeboid cells wander around make spicules. Check how These Beautiful Pictures of Different Types of. To be optimal for bathing, increasing with examples of brooding forms tan ct et al ratios derived from other microscopic plants from synthetic sponges belong to the university. What is those natural marine sponge? Different types of sponges come under different price points and loss different uses in. Global Diversity of Sponges Porifera NCBI NIH. Sponges EnchantedLearningcom. They publish the outer shape of rubber sponge 1 Some examples of sponges are Sea SpongeTube SpongeVase Sponge or Sponge Painted. Learn facts about the Porifera or Sea Sponges with our this Easy mountain for Kids. What claim a course Sponge Acme Sponge Company. BG Silicon isotopes of this sea sponges new insights into. Sponges come across an incredible summary of colors and an amazing array of shapes. 5 Fascinating Types of what Sponge Leisure Pro. Sea sponges often a tube-like bodies with his tiny pores. Sponges The World's Simplest Multi-Cellular Creatures. Sponges are food of various nudbranchs sea stars and fish. Examples of sponges Answers Answerscom. Sponges info and games Sheppard Software. -

Eurochordata and Cephalochordata of Persian Gulf & Oman See
Subphylum Urochordata : Tunicates Class Ascidacea Class Larvacea Class Thaliacea Subphylum Cephalochordata : Amphioxus Phylum Chordata : 1 - Subphylum Urochordata 2 Subphylum Cephalochordata (Subphylum Urochordata : Tunicates ) Class Ascidiacea Class Larvacea Class Thaliacea (Subphylum Cephalochordata ) Class Ascidiacea Ascidia Kowalevsky Patricia Kott D. lane David George & Gordon Paterson Herdmania momus savigny Herdmania momus kiamanensis Didemnum candidum savigny Phallusia nigra savigny Styela canopus savigny Class Ascidiacea : Order : Aplousobranchia Family : Polyclinidae : Aplidium cf. rubripunctum C.Monniot & F. Monniot 1997 Polyclinum constellatum Savigny, 1816 Family Didemnidae Didemnum candidum Savigny, 1816 Didemnum cf. granulatum Tokioka, 1954 Didemnum obscurum F. Monniot 1969 Didemnum perlucidum F. Monniot, 1983 Didemnum sp. Didemnum yolky C.Monniot & F. Monniot 1997 Diplosoma listerianum Milne Edwards, 1841 Order : Phlebobranchia Family : Ascidiidae Ascidia sp. Phallusia julinea Sluiter, 1915 Phallusia nigra Savigny, 1816 Order : Stolidobranchia Family : Styelidae Botryllus gregalis Sluiter, 1898 Botryllus niger Herdman, 1886 Botryllus sp. Eusynstyela cf. hartmeyeri Michaelsen, 1904 Polyandrocarpa sp. Styela canopus Savigny, 1816 Symplegma bahraini C.Monniot & F. Monniot 1997 Symplegma brakenhielmi Michaelsen, 1904 Family pyuridae Herdmania momus Savigny, 1816 Pyura curvigona Tokioka, 1950 Pyurid sp. Class Larvacea Larva Seymour Sewell Phylum Chordata Subphylum Urochordata * Class Larvacea Order Copelata Family Fritillariidae -

Phylum Arthropod Silvia Rondon, and Mary Corp, OSU Extension Entomologist and Agronomist, Respectively Hermiston Research and Extension Center, Hermiston, Oregon
Phylum Arthropod Silvia Rondon, and Mary Corp, OSU Extension Entomologist and Agronomist, respectively Hermiston Research and Extension Center, Hermiston, Oregon Member of the Phyllum Arthropoda can be found in the seas, in fresh water, on land, or even flying freely; a group with amazing differences of structure, and so abundant that all the other animals taken together are less than 1/6 as many as the arthropods. Well-known members of this group are the Kingdom lobsters, crayfish and crabs; scorpions, spiders, mites, ticks, Phylum Phylum Phylum Class the centipedes and millipedes; and last, but not least, the Order most abundant of all, the insects. Family Genus The Phylum Arthropods consist of the following Species classes: arachnids, chilopods, diplopods, crustaceans and hexapods (insects). All arthropods possess: • Exoskeleton. A hard protective covering around the outside of the body (divided by sutures into plates called sclerites). An insect's exoskeleton (integument) serves as a protective covering over the body, but also as a surface for muscle attachment, a water-tight barrier against desiccation, and a sensory interface with the environment. It is a multi-layered structure with four functional regions: epicuticle (top layer), procuticle, epidermis, and basement membrane. • Segmented body • Jointed limbs and jointed mouthparts that allow extensive specialization • Bilateral symmetry, whereby a central line can divide the body Insect molting or removing its into two identical halves, left and right exoesqueleton • Ventral nerve