Comparative Assessment of Carbon Stocks of Natural and Plantation Forest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
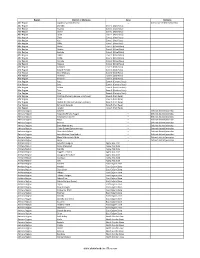
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Challenges of Clinical Chemistry Analyzers Utilization in Public Hospital Laboratories of Selected Zones of Oromia Region, Ethiopia: a Mixed Methods Study
Research Article ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2021.34.005584 Challenges of Clinical Chemistry Analyzers Utilization in Public Hospital Laboratories of Selected Zones of Oromia Region, Ethiopia: A Mixed Methods Study Rebuma Belete1*, Waqtola Cheneke2, Aklilu Getachew2 and Ahmedmenewer Abdu1 1Department of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia 2School of Medical Laboratory Sciences, Faculty of Health Sciences, Institute of Health, Jimma University, Jimma, Ethiopia *Corresponding author: Rebuma Belete, Department of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia ARTICLE INFO ABSTRACT Received: March 16, 2021 Background: The modern practice of clinical chemistry relies ever more heavily on automation technologies. Their utilization in clinical laboratories in developing countries Published: March 22, 2021 is greatly affected by many factors. Thus, this study was aimed to identify challenges affecting clinical chemistry analyzers utilization in public hospitals of selected zones of Oromia region, Ethiopia. Citation: Rebuma Belete, Waqtola Cheneke, Aklilu Getachew, Ahmedmenew- Methods: A cross-sectional study using quantitative and qualitative methods er Abdu. Challenges of Clinical Chemistry was conducted in 15 public hospitals from January 28 to March 15, 2019. Purposively Analyzers Utilization in Public Hospital selected 68 informants and 93 laboratory personnel working in clinical chemistry section Laboratories of Selected Zones of Oromia were included in the study. Data were collected by self-administered questionnaires, Region, Ethiopia: A Mixed Methods Study. in-depth interviews and observational checklist. The quantitative data were analyzed Biomed J Sci & Tech Res 34(4)-2021. by descriptive statistics using SPSS 25.0 whereas qualitative data was analyzed by a BJSTR. -

Aethiopica 19 (2016) International Journal of Ethiopian and Eritrean Studies
Aethiopica 19 (2016) International Journal of Ethiopian and Eritrean Studies ________________________________________________________________ MINAKO ISHIHARA, Nanzan University Article Change in the Significance of Affiliation to Ṭarīqa The Case of Tiǧāniyya in and around Ǧimma Aethiopica 19 (2016), 149–164 ISSN: 2194–4024 ________________________________________________________________ Edited in the Asien-Afrika-Institut Hiob Ludolf Zentrum für Äthiopistik der Universität Hamburg Abteilung für Afrikanistik und Äthiopistik by Alessandro Bausi in cooperation with Bairu Tafla, Ulrich Braukämper, Ludwig Gerhardt, Hilke Meyer-Bahlburg and Siegbert Uhlig Editorial The present issue of AETHIOPICA, like the preceding one, is partly monograph- ic, with a section containing the proceedings of the Panel on Islamic Literature in Ethiopia: New Perspectives of Research, from the ‘19th International Con- ference of Ethiopian Studies’, held in Warsaw, Poland, on 24–28 August 2015. Starting from this issue, the annual bibliography on Ethiopian Semitic and Cushitic linguistics held from its inception in 1998 for eighteen years by Rainer Voigt is handed over, on Voigt’s own will, to a pool of younger scholars, with the substantial support of the AETHIOPICA editorial team. I would like on this occasion to express the deep gratitude of the editorial board of AETHIOPICA and of all scholars in Ethiopian Semitic and Cushitic linguistics to Rainer Voigt for his fundamental and valuable contribution. Bibliographical abbreviations used in this volume AÉ Annales d’Éthiopie, Paris 1955ff. ÄthFor Äthiopistische Forschungen, 1–35, ed. by E. HAMMERSCHMIDT, 36–40, ed. by S. UHLIG (Stuttgart: Franz Steiner (1–34), 1977–1992; Wiesbaden: Harrassowitz (35–40), 1994–1995). AethFor Aethiopistische Forschungen, 41–73, ed. by S. -

Historical Survey of Limmu Genet Town from Its Foundation up to Present
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 6, ISSUE 07, JULY 2017 ISSN 2277-8616 Historical Survey Of Limmu Genet Town From Its Foundation Up To Present Dagm Alemayehu Tegegn Abstract: The process of modern urbanization in Ethiopia began to take shape since the later part of the nineteenth century. The territorial expansion of emperor Menelik (r. 1889 –1913), political stability and effective centralization and bureaucratization of government brought relative acceleration of the pace of urbanization in Ethiopia; the improvement of the system of transportation and communication are identified as factors that contributed to this new phase of urban development. Central government expansion to the south led to the appearance of garrison centers which gradually developed to small- sized urban center or Katama. The garrison were established either on already existing settlements or on fresh sites and also physically they were situated on hill tops. Consequently, Limmu Genet town was founded on the former Limmu Ennarya state‘s territory as a result of the territorial expansion of the central government and system of administration. Although the history of the town and its people trace many year back to the present, no historical study has been conducted on. Therefore the aim of this study is to explore the history of Limmu Genet town from its foundation up to present. Keywords: Limmu Ennary, Limmu Genet, Urbanization, Development ———————————————————— 1. Historical Background of the Study Area its production. The production and marketing of forest coffee spread the fame and prestige of Limmu Enarya ( The early history of Limmu Oromo Mohammeed Hassen, 1994). The name Limmu Ennarya is The history of Limmu Genet can be traced back to the rise derived from a combination of the name of the medieval of the Limmu Oromo clans, which became kingdoms or state of Ennarya and the Oromo clan name who settled in states along the Gibe river basin. -

Ethiopia Bellmon Analysis 2015/16 and Reassessment of Crop
Ethiopia Bellmon Analysis 2015/16 And Reassessment Of Crop Production and Marketing For 2014/15 October 2015 Final Report Ethiopia: Bellmon Analysis - 2014/15 i Table of Contents Acknowledgements ................................................................................................................................................ iii Table of Acronyms ................................................................................................................................................. iii Executive Summary ............................................................................................................................................... iv Introduction ................................................................................................................................................................ 9 Methodology .................................................................................................................................................. 10 Economic Background ......................................................................................................................................... 11 Poverty ............................................................................................................................................................. 14 Wage Labor ..................................................................................................................................................... 15 Agriculture Sector Overview ............................................................................................................................ -

(Coffea Arabica L.) Accessions Collected from Limmu Coffee
American Journal of BioScience 2021; 9(3): 79-85 http://www.sciencepublishinggroup.com/j/ajbio doi: 10.11648/j.ajbio.20210903.12 ISSN: 2330-0159 (Print); ISSN: 2330-0167 (Online) Phenotypic Diversity of Ethiopian Coffee ( Coffea arabica L.) Accessions Collected from Limmu Coffee Growing Areas Using Multivariate Analysis Lemi Beksisa *, Tadesse Benti, Getachew Weldemichael Ethiopian Institute of Agricultural Research, Jimma Agricultural Research Center, Jimma, Ethiopia Email address: *Corresponding author To cite this article: Lemi Beksisa, Tadesse Benti, Getachew Weldemichael. Phenotypic Diversity of Ethiopian Coffee ( Coffea arabica L.) Accessions Collected from Limmu Coffee Growing Areas Using Multivariate Analysis. American Journal of BioScience . Vol. 9, No. 3, 2021, pp. 79-85. doi: 10.11648/j.ajbio.20210903.12 Received : April 17, 2021; Accepted : May 11, 2021; Published : May 20, 2021 Abstract: Forty seven Coffea arabica L. germplasm accessions collected from Limmu district were field evaluated from 2004/5 to 2013/14 with two commercial check varieties at Agaro Agricultural Research sub center in single plot. The objective of the experiment was to assess the variability among the accessions using quantitative traits. Data for about eight quantitative traits were recorded only once in experimental period, while the yield data were recorded for six consecutive cropping seasons. Cluster, genetic divergence, and principal component analysis were used to assess the variability among the genotypes. The results revealed that average linkage cluster analysis for nine traits grouped the germplasm accessions in to three clusters. The number of accessions per cluster ranged from three in cluster III to 25 in cluster II. The clustering pattern of the coffee accessions revealed that the prevalence of moderate genetic diversity in Limmu coffee for the characters studied. -

Prioritization of Shelter/NFI Needs
Prioritization of Shelter/NFI needs Date: 31st May 2018 Shelter and NFI Needs As of 18 May 2018, the overall number of displaced people is 345,000 households. This figure is based on DTM round 10, partner’s assessments, government requests, as well as the total of HH supported since July 2017. The S/NFI updated its prioritisation in early May and SNFI Cluster partners agreed on several criteria to guide prioritisation which include: - 1) type of emergency, 2) duration of displacement, and 3) sub-standard shelter conditions including IDPS hosted in collective centres and open-air sites and 4) % of vulnerable HH at IDP sites. Thresholds for the criteria were also agreed and in the subsequent analysis the cluster identified 193 IDP hosting woredas mostly in Oromia and Somali regions, as well as Tigray, Gambella and Addis Ababa municipality. A total of 261,830 HH are in need of urgent shelter and NFI assistance. At present the Cluster has a total of 57,000 kits in stocks and pipeline. The Cluster requires urgent funding to address the needs of 204,830 HHs that are living in desperate displacement conditions across the country. This caseload is predicted to increase as the flooding continues in the coming months. Shelter and NFI Priority Activities In terms of priority activities, the SNFI Cluster is in need of ES/NFI support for 140,259 HH displaced mainly due to flood and conflict under Pillar 2, primarily in Oromia and Somali Regions. In addition, the Shelter and NFI Cluster requires immediate funding for recovery activities to support 14,000 HH (8,000 rebuild and 6,000 repair) with transitional shelter support and shelter repair activities under Pillar 3. -

Ethiopia Round 6 SDP Questionnaire
Ethiopia Round 6 SDP Questionnaire Always 001a. Your name: [NAME] Is this your name? ◯ Yes ◯ No 001b. Enter your name below. 001a = 0 Please record your name 002a = 0 Day: 002b. Record the correct date and time. Month: Year: ◯ TIGRAY ◯ AFAR ◯ AMHARA ◯ OROMIYA ◯ SOMALIE BENISHANGUL GUMZ 003a. Region ◯ ◯ S.N.N.P ◯ GAMBELA ◯ HARARI ◯ ADDIS ABABA ◯ DIRE DAWA filter_list=${this_country} ◯ NORTH WEST TIGRAY ◯ CENTRAL TIGRAY ◯ EASTERN TIGRAY ◯ SOUTHERN TIGRAY ◯ WESTERN TIGRAY ◯ MEKELE TOWN SPECIAL ◯ ZONE 1 ◯ ZONE 2 ◯ ZONE 3 ZONE 5 003b. Zone ◯ ◯ NORTH GONDAR ◯ SOUTH GONDAR ◯ NORTH WELLO ◯ SOUTH WELLO ◯ NORTH SHEWA ◯ EAST GOJAM ◯ WEST GOJAM ◯ WAG HIMRA ◯ AWI ◯ OROMIYA 1 ◯ BAHIR DAR SPECIAL ◯ WEST WELLEGA ◯ EAST WELLEGA ◯ ILU ABA BORA ◯ JIMMA ◯ WEST SHEWA ◯ NORTH SHEWA ◯ EAST SHEWA ◯ ARSI ◯ WEST HARARGE ◯ EAST HARARGE ◯ BALE ◯ SOUTH WEST SHEWA ◯ GUJI ◯ ADAMA SPECIAL ◯ WEST ARSI ◯ KELEM WELLEGA ◯ HORO GUDRU WELLEGA ◯ Shinile ◯ Jijiga ◯ Liben ◯ METEKEL ◯ ASOSA ◯ PAWE SPECIAL ◯ GURAGE ◯ HADIYA ◯ KEMBATA TIBARO ◯ SIDAMA ◯ GEDEO ◯ WOLAYITA ◯ SOUTH OMO ◯ SHEKA ◯ KEFA ◯ GAMO GOFA ◯ BENCH MAJI ◯ AMARO SPECIAL ◯ DAWURO ◯ SILTIE ◯ ALABA SPECIAL ◯ HAWASSA CITY ADMINISTRATION ◯ AGNEWAK ◯ MEJENGER ◯ HARARI ◯ AKAKI KALITY ◯ NEFAS SILK-LAFTO ◯ KOLFE KERANIYO 2 ◯ GULELE ◯ LIDETA ◯ KIRKOS-SUB CITY ◯ ARADA ◯ ADDIS KETEMA ◯ YEKA ◯ BOLE ◯ DIRE DAWA filter_list=${level1} ◯ TAHTAY ADIYABO ◯ MEDEBAY ZANA ◯ TSELEMTI ◯ SHIRE ENIDASILASE/TOWN/ ◯ AHIFEROM ◯ ADWA ◯ TAHTAY MAYCHEW ◯ NADER ADET ◯ DEGUA TEMBEN ◯ ABIYI ADI/TOWN/ ◯ ADWA/TOWN/ ◯ AXUM/TOWN/ ◯ SAESI TSADAMBA ◯ KLITE -

Developing the Butter Value Chain in Ethiopia
LIVES WORKING PAPER 1 PAPER WORKING LIVES Developing the butter value chain in Ethiopia ISBN 92–9146–366–3 Livestock and irrigation value chains for Ethiopian smallholders project aims to improve the competitiveness, sustainability and equity of value chains for selected high‐value livestock and irrigated crop commodities in target areas of four regions of Ethiopia. It identifies, targets and promotes improved technologies and innovations to develop high value livestock and irrigated crop value chains; it improves the capacities of Livestock and Irrigation Value chains for Ethiopian Smallholders value chain actors; it improves the use of knowledge at different levels; it generates knowledge through action‐oriented research; and it promotes and disseminates good practices. Project carried out with the financial support of the Government of Canada provided through Foreign Affairs, Trade and Development Canada (DFATD). lives-ethiopia.org The International Livestock Research Institute (ILRI) works to improve food security and reduce poverty in developing countries through research for better and more sustainable use of livestock. ILRI is a member of the CGIAR Consortium, a global research partnership of 15 centres working with many partners for a food-secure future. ILRI has two main campuses in East Africa and other hubs in East, West and southern Africa and South, Southeast and East Asia. ilri.org The International Water Management Institute (IWMI) is a non-profit, scientific research organization focusing on the sustainable use of water and land resources in developing countries. It is headquartered in Colombo, Sri Lanka, with regional offices across Asia and Africa. IWMI works in partnership with governments, civil society and the private sector to develop scalable agricultural water management solutions that have a real impact on poverty reduction, food security and ecosystem health. -

The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia
The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia A Case Study from Jimma Area Aklilu Amsalu, with Eva Ludi NCCR North-South Dialogue, no. 26 2010 The present study was carried out at the following partner institutions of the NCCR North-South: Overseas Development Institution (ODI) London, UK Department of Geography & Environmental Studies Addis Ababa University, Ethiopia Regional Coordination Office, JACS East Africa Addis Abeba, Ethiopia Swisspeace Bern, Switzerland The NCCR North-South (Research Partnerships for Mitigating Syndromes of Global Change) is one of twenty National Centres of Competence in Research established by the Swiss National Science Foundation (SNSF). It is implemented by the SNSF and co- funded by the Swiss Agency for Development and Cooperation (SDC), and the participating institutions in Switzerland. The NCCR North-South carries out disciplinary, interdisciplinary and transdisciplinary research on issues relating to sustainable development in developing and transition countries as well as in Switzerland. http://www.north-south.unibe.ch The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia A Case Study from Jimma Area Aklilu Amsalu, with Eva Ludi NCCR North-South Dialogue, no. 26 2010 Citation Aklilu Amsalu, Ludi E. 2010. The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia: A Case Study from Jimma Area. NCCR North-South Dialogue 26. Bern, Switzerland: NCCR North-South. Editing Stefan Zach, z.a.ch gmbh, Switzerland Cover photos Left: Typical landscape in the Jimma area – a mosaic of coffee forests and crop land. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

Integrating Food Security and Biodiversity Governance a Multi
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/326632264 Integrating food security and biodiversity governance: A multi-level social network analysis in Ethiopia Article in Land Use Policy · July 2018 DOI: 10.1016/j.landusepol.2018.07.014 CITATION READS 1 133 4 authors: Tolera Senbeto Jiren Arvid Bergsten Leuphana University Lüneburg Leuphana University Lüneburg 5 PUBLICATIONS 2 CITATIONS 15 PUBLICATIONS 122 CITATIONS SEE PROFILE SEE PROFILE Julia Leventon Joern Fischer Leuphana University Lüneburg Leuphana University Lüneburg 38 PUBLICATIONS 483 CITATIONS 220 PUBLICATIONS 13,108 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Wood-pastures of Transylvania (Romania). View project Is informal labor sharing among smallholders influencing their food security and crop-choice sovereignty? View project All content following this page was uploaded by Tolera Senbeto Jiren on 09 August 2018. The user has requested enhancement of the downloaded file. Land Use Policy 78 (2018) 420–429 Contents lists available at ScienceDirect Land Use Policy journal homepage: www.elsevier.com/locate/landusepol Integrating food security and biodiversity governance: A multi-level social network analysis in Ethiopia T ⁎ Tolera Senbeto Jiren , Arvid Bergsten, Ine Dorresteijn, Neil French Collier, Julia Leventon, Joern Fischer Leuphana University, Faculty of Sustainability, Scharnhorststrasse 1, D21335 Luneburg, Germany ARTICLE INFO ABSTRACT Keywords: Integrating food security and biodiversity conservation is an important contemporary challenge. Traditionally, Biodiversity food security and biodiversity conservation have been considered as separate or even incompatible policy goals. Food security However, there is growing recognition of their interdependence, as well as of the need to coordinate solutions Governance across multiple policy sectors and levels of governance.