Challenges of Clinical Chemistry Analyzers Utilization in Public Hospital Laboratories of Selected Zones of Oromia Region, Ethiopia: a Mixed Methods Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

Situational Analysis of Child Sexual Abuse and Exploitation: the Case of Jimma and Agaro Towns of Oromia Regional State
European Scientific Journal September 2014 edition vol.10, No.26 ISSN: 1857 – 7881 (Print) e - ISSN 1857- 7431 SITUATIONAL ANALYSIS OF CHILD SEXUAL ABUSE AND EXPLOITATION: THE CASE OF JIMMA AND AGARO TOWNS OF OROMIA REGIONAL STATE Gudina Abashula Nega Jibat Jimma University, College of Social Sciences and Humanities, Department of Sociology and Social Work Abstract This study was conducted to assess people’s awareness, patterns, community responses and factors for child sexual abuse and exploitation in Jimma and Agaro town. Survey, key informant interview and case studies were used to collect the information required for the study. The data collected was analyzed using percentages and case studies under themes developed based on the research objectives. The findings of the study revealed that eighty nine percent of the respondents are aware of the existence of child sexual abuse in their community and more than half of them have identified sexual intercourse with children, child pornography and child genital stimulation as child sexual abuses. Both girls and boys can be exposed to child sexual abuse; however, girls are more vulnerable to child sexual abuses than boys. In terms of their backgrounds, street children, orphans and children of the poor families are mainly vulnerable to child sexual abuse. Children are sexually abused in their home, in the community and in organizations as the findings from the case studies and key informant interviews indicated. Death of parents, family poverty and the subsequent inability to fulfill the necessary basic needs for children; abusers’ perception about sexual affairs with children is safe as they think children are free from HIV/AIDS, lack of appropriate care and follow up for children are the major factors for child sexual abuse. -

Midterm Survey Protocol
Protocol for L10K Midterm Survey The Last 10 Kilometers Project JSI Research & Training Institute, Inc. Addis Ababa, Ethiopia October 2010 Contents Introduction ........................................................................................................................................................ 2 The Last Ten Kilometers Project ............................................................................................................ 3 Objective one activities cover all the L10K woredas: .......................................................................... 4 Activities for objectives two, three and four in selected woredas ...................................................... 5 The purpose of the midterm survey ....................................................................................................... 6 The midterm survey design ...................................................................................................................... 7 Annex 1: List of L10K woredas by region, implementation strategy, and implementing phase ......... 10 Annex 2: Maps.................................................................................................................................................. 11 Annex 3: Research questions with their corresponding study design ...................................................... 14 Annex 4: Baseline survey methodology ........................................................................................................ 15 Annex 5: L10K midterm survey -

Ethiopia Bellmon Analysis 2015/16 and Reassessment of Crop
Ethiopia Bellmon Analysis 2015/16 And Reassessment Of Crop Production and Marketing For 2014/15 October 2015 Final Report Ethiopia: Bellmon Analysis - 2014/15 i Table of Contents Acknowledgements ................................................................................................................................................ iii Table of Acronyms ................................................................................................................................................. iii Executive Summary ............................................................................................................................................... iv Introduction ................................................................................................................................................................ 9 Methodology .................................................................................................................................................. 10 Economic Background ......................................................................................................................................... 11 Poverty ............................................................................................................................................................. 14 Wage Labor ..................................................................................................................................................... 15 Agriculture Sector Overview ............................................................................................................................ -

Characterization of Livestock Production System in Three Selected Districts of Jimma Zone, Southwest Ethiopia
Journal of Reproduction and Infertility 7 (2): 47-62, 2016 ISSN 2079-2166 © IDOSI Publications, 2016 DOI: 10.5829/idosi.jri.2016.7.2.102165 Characterization of Livestock Production System in Three Selected Districts of Jimma Zone, Southwest Ethiopia 12Mohammed Husen, Yisehak Kechero and 3Meseret Molla 1Ethiopian Agricultural Transformation Agency, P.O. Box: 708, Addis Ababa, Ethiopia 2Department of Animal Sciences, Arba Minch University, Arba Minch, Ethiopia 3Department of Animal Sciences, Jimma University, P.O. Box: 307, Jimma, Ethiopia Abstract: The cross-sectional field survey was conducted in three selected districts of Jimma zone with the aim of characterizing the prevailing livestock production systems as well as identifying the major constraints and opportunities in relation to livestock production. The study districts were selected based on their livestock production potential and accessibility. Accordingly, 122, 188 and 104 households (HHs) from Kersa, Omo Nada and Tiro Afeta districts, respectively were participated in the study. The respondent HHs were purposively selected depending on their livestock keeping experience. This study revealed that livestock production systems in the three districts were mixed crop-livestock production system which is totally based on the indigenous livestock breeds with no improved input and low output. The average number of livestock in terms of tropical livestock units (TLU) in the three districts was 5.10±0.32/HH, which varied significantly (P<0.01) between the districts. Overall, the herd structure comprised of cattle (4.74±0.24 TLU/HH) (P<0.05), sheep (0.10±0.01 TLU/HH), goats (0.06±0.01 HH), donkey (0.07±0.02 TLU/HH) (P<0.05), horses (0.05±0.02 TLU/HH) and mule (0.06±0.03 TLU/HH). -

The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia
The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia A Case Study from Jimma Area Aklilu Amsalu, with Eva Ludi NCCR North-South Dialogue, no. 26 2010 The present study was carried out at the following partner institutions of the NCCR North-South: Overseas Development Institution (ODI) London, UK Department of Geography & Environmental Studies Addis Ababa University, Ethiopia Regional Coordination Office, JACS East Africa Addis Abeba, Ethiopia Swisspeace Bern, Switzerland The NCCR North-South (Research Partnerships for Mitigating Syndromes of Global Change) is one of twenty National Centres of Competence in Research established by the Swiss National Science Foundation (SNSF). It is implemented by the SNSF and co- funded by the Swiss Agency for Development and Cooperation (SDC), and the participating institutions in Switzerland. The NCCR North-South carries out disciplinary, interdisciplinary and transdisciplinary research on issues relating to sustainable development in developing and transition countries as well as in Switzerland. http://www.north-south.unibe.ch The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia A Case Study from Jimma Area Aklilu Amsalu, with Eva Ludi NCCR North-South Dialogue, no. 26 2010 Citation Aklilu Amsalu, Ludi E. 2010. The Effect of Global Coffee Price Changes on Rural Livelihoods and Natural Resource Management in Ethiopia: A Case Study from Jimma Area. NCCR North-South Dialogue 26. Bern, Switzerland: NCCR North-South. Editing Stefan Zach, z.a.ch gmbh, Switzerland Cover photos Left: Typical landscape in the Jimma area – a mosaic of coffee forests and crop land. -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

Diversity, Composition and Nutrient Adequacy of Diets of Lactating Mothers in Jimma Zone, Southwest Ethiopia
Tropentag 2016, Vienna, Austria September 18-21, 2016 Conference on International Research on Food Security, Natural Resource Management and Rural Development organised by the University of Natural Resources and Life Sciences (BOKU Vienna), Austria Fighting Hidden Hunger: Diversity, Composition and Nutrient Adequacy of Diets of Lactating Mothers in Jimma Zone, Southwest Ethiopia Sirawdink F. Forsidoac, Tefera Belachewb and Oliver Henselc a Department of Post-Harvest Management, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia b Department of Population and Family Health, Nutrition Unit, College of Public Health and Medical Sciences, Jimma University, Jimma, Ethiopia c Department of Agricultural and Bioprocess Engineering, Faculty of Organic Agricultural Sciences, University of Kassel, Witzenhausen, Germany Introduction Maternal and child undernutrition is amongst the major challenges of our times. It has been on the global agenda as central to health, sustainable development, and progress (Black et al., 2008). Maternal and child undernutrition and micronutrient deficiencies affect approximately half of the world’s population. The human, social and economic costs to society at large are enormous in terms of lost productivity, health, and well-being, decreased learning ability and reduced fulfilment of human potential (Ahmed, Hossain, & Sanin, 2012). Mothers from low-income settings are amongst the most vulnerable to undernutrition because their body’s nutrient reserve is severely affected due to frequent pregnancies and lactation (Haileslassie, Mulugeta, & Girma, 2013). According to UNICEF’s conceptual framework of undernutrition, inadequate dietary intake is an immediate cause of maternal and child undernutrition (Black et al., 2008). The study area, Jimma zone, is year round green but unfortunately characterized by household food insecurity (Belachew et al., 2013) and information on the diversity, composition and nutrient adequacy of diets consumed by mothers during lactation period is lacking. -
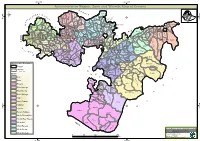
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Governing the Faithful
Ethiopia: Governing the Faithful Crisis Group Africa Briefing N°117 Nairobi/Brussels, 22 February 2016 I. Overview Ethiopia provides a significant example of the struggle governments are undertaking to find and implement effective policy responses to faith-based violent extremism and sectarian conflict. Given both demographic shifts and greater religious freedoms, the management of religious conflict and practice has of necessity been a complex and sometimes fraught task. A changed context has seen the Ethiopian People’s Revolu- tionary Democratic Front (EPRDF) government shift from mediating conflict between faith groups to regulating religious practice, especially where there are political or extremist overtones. Local actors have used the state’s interventionist inclinations in the confessional realm to gain advantage in wider leadership struggles within Ethio- pia’s ethnically based regional states. Opposition groups, not always within formal par- ties, have also used religious issues to score political points. The Ethiopian experience shows not only how faith is an increasing political resource, especially at local levels, but also lessons that can be learned from top-down interventions in the religious sphere. Although often regarded as a predominantly Christian country, the confessional landscape is diverse and evolving, and religion is increasingly politicised by a range of domestic actors, including the state. Faith runs deep, and its religions (particularly the Orthodox Church) have at various times in history been intimately connected to the Ethiopian state and its administration. Always a significant but institutionally dis- advantaged minority, the Muslim population has grown in relative terms in recent decades and is at least as numerous as that in Sudan, Ethiopia’s predominantly Islamic neighbour. -

Ethiopia: the State of the World's Forest Genetic Resources
ETHIOPIA This country report is prepared as a contribution to the FAO publication, The Report on the State of the World’s Forest Genetic Resources. The content and the structure are in accordance with the recommendations and guidelines given by FAO in the document Guidelines for Preparation of Country Reports for the State of the World’s Forest Genetic Resources (2010). These guidelines set out recommendations for the objective, scope and structure of the country reports. Countries were requested to consider the current state of knowledge of forest genetic diversity, including: Between and within species diversity List of priority species; their roles and values and importance List of threatened/endangered species Threats, opportunities and challenges for the conservation, use and development of forest genetic resources These reports were submitted to FAO as official government documents. The report is presented on www. fao.org/documents as supportive and contextual information to be used in conjunction with other documentation on world forest genetic resources. The content and the views expressed in this report are the responsibility of the entity submitting the report to FAO. FAO may not be held responsible for the use which may be made of the information contained in this report. THE STATE OF FOREST GENETIC RESOURCES OF ETHIOPIA INSTITUTE OF BIODIVERSITY CONSERVATION (IBC) COUNTRY REPORT SUBMITTED TO FAO ON THE STATE OF FOREST GENETIC RESOURCES OF ETHIOPIA AUGUST 2012 ADDIS ABABA IBC © Institute of Biodiversity Conservation (IBC) -

Study on Rumen and Reticulum Foreign Bodies in Cattle Slaughtered at Jimma Municipal Abattoir, South West Ethiopia
American-Eurasian Journal of Scientific Research 7 (4): 160-167, 2012 ISSN 1818-6785 © IDOSI Publications, 2012 DOI: 10.5829/idosi.aejsr.2012.7.4.65140 Study on Rumen and Reticulum Foreign Bodies in Cattle Slaughtered at Jimma Municipal Abattoir, South West Ethiopia Desiye Tesfaye and Mersha Chanie University of Gondar, Faculty of Veterinary Medicine, Department of Paraclinical Studies, P.O.Box, 196. Gondar, Ethiopia Abstract: A cross-sectional study was conducted from October, 2011 to March, 2012 at Jimma Municipal Abattoir, Oromia Regional State, Southwest Ethiopia, with the objectives of to assess the prevalence of rumen and reticulum foreign bodies, identifying types of foreign bodies and associated risk factors for the occurrences of foreign bodies. Postmortem examination was employed for the recovery of foreign body from rumen and reticulum. The investigation was carried out in the abattoir. From total of 484 (464 male and 20 female) cattle were examined, 13.22 %( n=64) were found foreign bodies at slaughter. When the prevalence was compared between sex, between breed, among different age groups, among different body condition score and animal originated from different areas, higher prevalence of foreign bodies 80%, 70%,80%,72.72%,25.23% were observed in female, cross breed, age older than 10 years, animal having poor body condition score and animal originate from Agaro, respectively. These aforementioned factors are considered as potential risk factors and found highly significantly associated (p < 0.05) with the occurrence of foreign bodies. Rumen harbored mostly plastic materials while reticulum was the major site for the retention of metallic objects. Plastics were recovered as the most common foreign bodies followed by leathers, clothes, ropes, nails and wires.