(12) Patent Application Publication (10) Pub. No.: US 2012/0184642 A1 Bartling Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

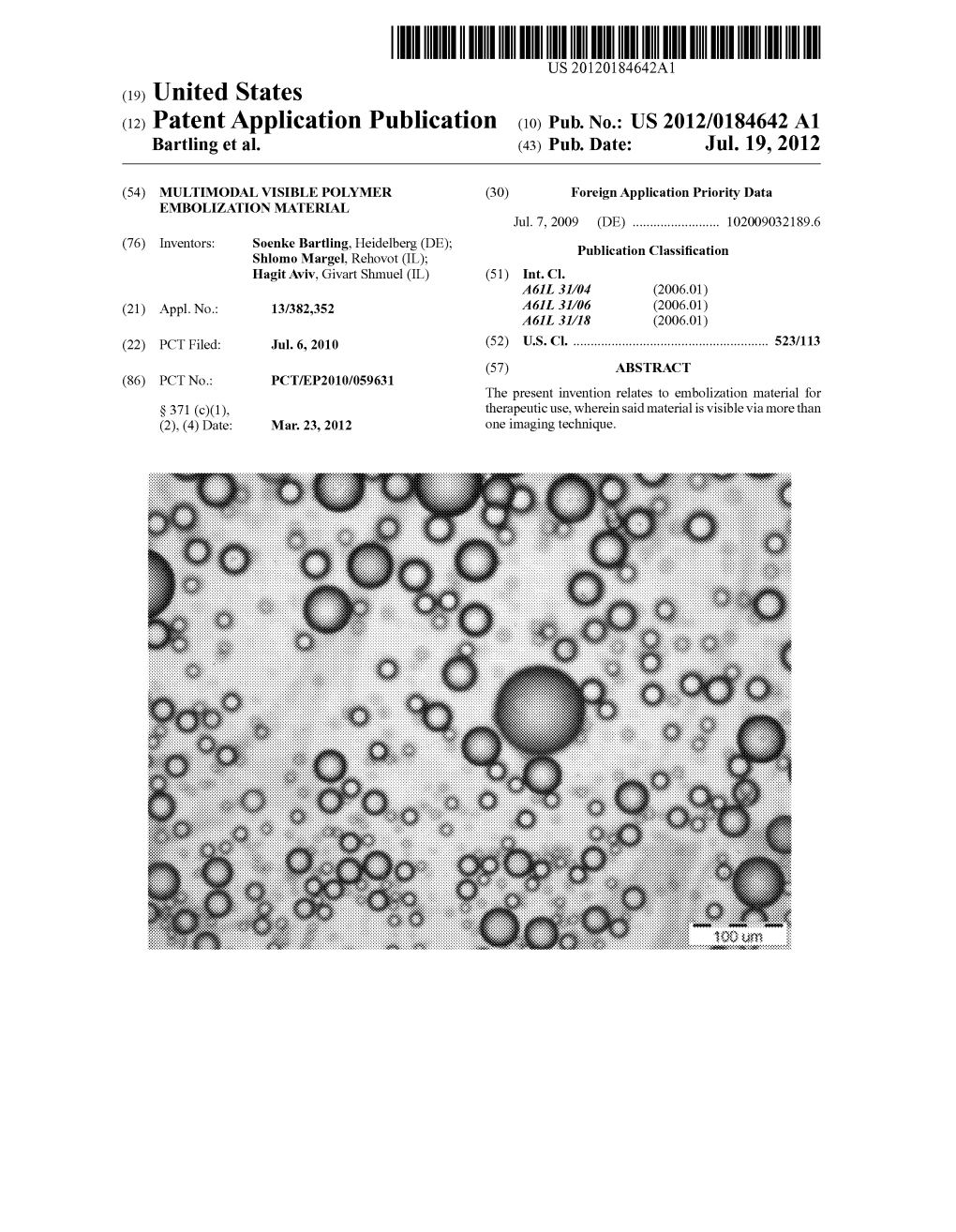
Mri Contrast Medium and Diagnostic Method
Europa.schesP— || | MMMMI 1 1||||| 1 1 1 1 1 1||| || J European Patent Office _ o it r- a 4 © Publication number: 0 673 655 A1 Office_„. europeen des brevets © EUROPEAN PATENT APPLICATION published in accordance with Art. 158(3) EPC © Application number: 93906789.8 © Int. CI.6: A61 K 49/00 @ Date of filing: 18.03.93 © International application number: PCT/JP93/00322 © International publication number: WO 93/18795 (30.09.93 93/24) ® Priority: 19.03.92 JP 93561/92 Kanagawa 211 (JP) Inventor: IWAI, Hlroyuki, Yodogawa Works of @ Date of publication of application: Daikin Ind.Ltd.s 27.09.95 Bulletin 95/39 1-1, Nlshlhltotsuya Settsu-shl, © Designated Contracting States: Osaka 566 566 (JP) DE FR GB IT Inventor: YAMASHITA, Tsuneo, Yodogawa Works © Applicant: DAIKIN INDUSTRIES, LIMITED of Daikin Ind. Ltd., Umeda Center Building, 4-12, Nakazaki-nishi 1-1, Nishihitotsuya 2-chome Settsu-shi, Kita-ku Osaka 566 566 (JP) Osaka-shi Inventor: SHIMOKAWA, Kazuhiro, Yodogawa Osaka 530 (JP) Works of Daikin Ind. Ltd., © Inventor: YOSHIKAWA, Kohki 1-1, Nishihitotsuya 14-23, Kami-saginomiya 5-chome, Settsu-shi, Nakano-ku Osaka 566 (JP) Tokyo 165 (JP) Inventor: SHIONO, Takahiro, Kanto Rohsai Hospital © Representative: TER MEER - MULLER - 2035, Kizuki Sumiyoshi-cho, STEINMEISTER & PARTNER Nakahara-ku Mauerkircherstrasse 45 Kawasaki-shi, D-81679 Munchen (DE) ^ © MRI CONTRAST MEDIUM AND DIAGNOSTIC METHOD. in m CO © An MRI contrast medium (suspension) comprising an oily fatty acid, fatty acid ester or perfluorinated compound and a particulate paramagnetic compound contained therein. It can be used stably for long and can ^ be administered to a target region, whereby its dose can be reduced. -

Sulfur Hexafluoride Hazard Summary Identification
Common Name: SULFUR HEXAFLUORIDE CAS Number: 2551-62-4 RTK Substance number: 1760 DOT Number: UN 1080 Date: April 2002 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Sulfur Hexafluoride can affect you when breathed in. * If you think you are experiencing any work-related health * Sulfur Hexafluoride can irritate the skin causing a rash or problems, see a doctor trained to recognize occupational burning feeling on contact. Direct skin contact can cause diseases. Take this Fact Sheet with you. frostbite. * Exposure to hazardous substances should be routinely * Sulfur Hexafluoride may cause severe eye burns leading evaluated. This may include collecting personal and area to permanent damage. air samples. You can obtain copies of sampling results * Breathing Sulfur Hexafluoride can irritate the nose and from your employer. You have a legal right to this throat. information under OSHA 1910.1020. * Breathing Sulfur Hexafluoride may irritate the lungs causing coughing and/or shortness of breath. Higher WORKPLACE EXPOSURE LIMITS exposures can cause a build-up of fluid in the lungs OSHA: The legal airborne permissible exposure limit (pulmonary edema), a medical emergency, with severe (PEL) is 1,000 ppm averaged over an 8-hour shortness of breath. workshift. * High exposure can cause headache, confusion, dizziness, suffocation, fainting, seizures and coma. NIOSH: The recommended airborne exposure limit is * Sulfur Hexafluoride may damage the liver and kidneys. 1,000 ppm averaged over a 10-hour workshift. * Repeated high exposure can cause deposits of Fluorides in the bones and teeth, a condition called “Fluorosis.” This ACGIH: The recommended airborne exposure limit is may cause pain, disability and mottling of the teeth. -

Caracterización Fisicoquímica Y Clínica De Los Medios De Contraste Intravasculares Iodados
Anales de Radiología México 2008;2:129-140. ARTÍCULOS DE REVISIÓN Dra. Patricia Rodríguez Nava,1 Dr. Ernesto J. Dena Espinoza,1 Caracterización fisicoquímica y Dr. Roberto Basile Lenge,2 Dra. Margarita Fuentes García,3 clínica de los medios de contraste Dr. Gustavo Fink Josephi,4 Dr. Eduardo Marbez Namnum1 intravasculares iodados RESUMEN Objetivo: El propósito de sido aceptado por algunos ra- este artículo es realizar una re- diólogos. La lopromida puede Introducción: El uso de visión bibliográfica sobre las ca- ser considerada un agente uni- medios de contraste intravascu- racterísticas, clasificación, pro- versal para todas las explora- lares no iónicos (gas, sustancia piedades físico-químicas, así ciones y procedimientos radio- hidrosoluble o liposoluble) en como los efectos secundarios lógicos. imagenología ha proliferado (quimiotoxicidad) de los medios en los últimos años debido a su de contraste intravasculares no Palabras clave: Medios de excepcional tolerancia por los iónicos. contraste intravasculares ioda- pacientes. La baja osmolalidad Conclusiones: Un factor que dos, medios de contraste ióni- de este tipo de medios de con- interviene en la incidencia de cos, medios de contraste no ió- traste aporta beneficios como reacciones idiosincráticas o aler- nicos. bajo incremento del volumen goides puede ser el estado psi- sanguíneo, baja toxicidad, bajo cológico del paciente. El trata- efecto sobre la barrera hema- miento previo con antihitamíni- toencefálica. cos, corticosteroides o ambos ha continúa en la pág. 130 1 Del Servicio de Radiología e Imagen “Dr. Carlos Coqui” del Hospital General de México osmolalidad o iso-osmolares. En los Estados Unidos 2 3 O.D. De la Universidad de Buenos Aires Cardiología. -

Drug-Induced Anaphylaxis in China: a 10 Year Retrospective Analysis of The
Int J Clin Pharm DOI 10.1007/s11096-017-0535-2 RESEARCH ARTICLE Drug‑induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database Ying Zhao1,2,3 · Shusen Sun4 · Xiaotong Li1,3 · Xiang Ma1 · Huilin Tang5 · Lulu Sun2 · Suodi Zhai1 · Tiansheng Wang1,3,6 Received: 9 May 2017 / Accepted: 19 September 2017 © The Author(s) 2017. This article is an open access publication Abstract Background Few studies on the causes of (50.1%), mucocutaneous (47.4%), and gastrointestinal symp- drug-induced anaphylaxis (DIA) in the hospital setting are toms (31.3%). A total of 249 diferent drugs were involved. available. Objective We aimed to use the Beijing Pharma- DIAs were mainly caused by antibiotics (39.3%), traditional covigilance Database (BPD) to identify the causes of DIA Chinese medicines (TCM) (11.9%), radiocontrast agents in Beijing, China. Setting Anaphylactic case reports from (11.9%), and antineoplastic agents (10.3%). Cephalospor- the BPD provided by the Beijing Center for Adverse Drug ins accounted for majority (34.5%) of antibiotic-induced Reaction Monitoring. Method DIA cases collected by the anaphylaxis, followed by fuoroquinolones (29.6%), beta- BPD from January 2004 to December 2014 were adjudi- lactam/beta-lactamase inhibitors (15.4%) and penicillins cated. Cases were analyzed for demographics, causative (7.9%). Blood products and biological agents (3.1%), and drugs and route of administration, and clinical signs and plasma substitutes (2.1%) were also important contributors outcomes. Main outcome measure Drugs implicated in DIAs to DIAs. Conclusion A variety of drug classes were impli- were identifed and the signs and symptoms of the DIA cases cated in DIAs. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

BGA244 Binary Gas Analyzer User Manual
BGA244 Binary Gas Analyzer User Manual Revision 1.10 Certification Stanford Research Systems certifies that this product met its published specification at the time of shipment. Warranty This Stanford Research Systems product is warranted against defects in materials and workmanship for a period of one (1) year from the date of shipment. Service For warranty service or repair, this product must be returned to a Stanford Research Systems authorized service facility. Contact Stanford Research Systems or an authorized representative for a RMA (Return Material Authorization) Number before returning this product for repair. These are available at www.thinksrs.com under Support, Repair/Calibration. All users returning a BGA244 back to the factory for repair and/or service must submit a correctly completed “Declaration of Contamination of Equipment” form, available as part of the RMA process. The SRS personnel carrying out repair and service of the BGA244 must be informed of the condition of the components prior to any work being performed. Warning All returns to SRS must be free of harmful, corrosive, radioactive or toxic materials. Dedication In memory of Jim Williams, 1948 - 2011: Legendary Author and Analog Design Guru Information in this document is subject to change without notice. Copyright © Stanford Research Systems, Inc., 2016-2018. All rights reserved. Stanford Research Systems, Inc. 1290-C Reamwood Avenue Sunnyvale, California 94089 Phone: (408) 744-9040 Fax: (408) 744-9049 Email: [email protected] www.thinksrs.com Printed in the -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

Chromatographic Analysis of Pharmaceuticals Second Edition, Revised and Expanded
Chromatographic Analysis of Pharmaceuticals Second Edition, Revised and Expanded edited by John A. Adamovics Cytogen Corporation Princeton, New Jersey Marcel Dekker, Inc. New York-Basel «Hong Kong Preface ISBN: 0-8247-9776-0 The first edition of Chromatographic Analysis of Pharmaceuticals was The publisher offers discounts on this book when ordered in bulk quanti published in 1990. The past years have allowed me to evaluate leads that I ties. For more information, write to Special Sales/Professional Marketing uncovered during the researching of the first edition, such as the first pub at the address below. lished example of the application of chromatography to pharmaceutical analysis of medicinal plants. This and other examples are found in a rela This book is printed on acid-free paper. tively rare book, Uber Kapillaranalyse und ihre Anwendung in Pharmazeu- tichen Laboratorium (Leipzig, 1992), by H. Platz. Capillary analysis, the Copyright © 1997 by Marcel Dekker, Inc. All Rights Reserved. chromatographic technique used, was developed by Friedlieb Runge in the mid-1850s and was later refined by Friedrich Goppelsroeder. The principle Neither this book nor any part may be reproduced or transmitted in any of the analysis was that substances were absorbed on filter paper directly form or by any means, electronic or mechanical, including photocopying, from the solutions in which they were dissolved; they then migrated to microfilming, and recording, or by any information storage and retrieval different points on the filter paper. Capillary analysis differed from paper system, without permission in writing from the publisher. chromatography in that no developing solvent was used. We find that, from these humble beginnings 150 years ago, the direct descendant of this Marcel Dekker, Inc. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

1488 Contrast Media
1488 Contrast Media Metrizoic Acid (BANM, rINNM) form microbubbles of perflenapent gas that provide echo- be diluted in 50 mL of sodium chloride 0.9% and given by intra- enhancement. A small amount of perflisopent (below) was also venous infusion at an initial rate of 4 mL/minute, adjusted as re- Acide Métrizoïque; Ácido metrizoico; Acidum Metrizoicum; included in the formulation. quired, to a maximum rate of 10 mL/minute. Metritsoiinihappo; Metrizoinsyra. 3-Acetamido-2,4,6-tri-iodo-5- (N-methylacetamido)benzoic acid. ◊ References. Serious cardiopulmonary reactions, including fatalities, have been reported with perflutren and it should be used with extreme Метризоевая Кислота 1. Robbin ML, Eisenfeld AJ. Perflenapent emulsion: a US contrast agent for diagnostic radiology—multicenter, double-blind com- caution in patients with pulmonary hypertension or unstable car- C12H11I3N2O4 = 627.9. parison with a placebo. Radiology 1998; 207: 717–22. diopulmonary conditions. The safety of perflutren has not been CAS — 1949-45-7. 2. Kitzman DW, Wesley DJ. Safety assessment of perflenapent established in patients with right-to-left cardiac shunts; it should ATC — V08AA02. emulsion for echocardiographic contrast enhancement in pa- also be used with extreme caution or avoided in such patients. tients with congestive heart failure or chronic obstructive pulmo- Perflutren has also been given by intra-ocular injection to pro- ATC Vet — QV08AA02. nary disease. Am Heart J 2000; 139: 1077–80. vide tamponade in the management of retinal detachment. Adverse effects. References. H C 3 1. Herzog CA. Incidence of adverse events associated with use of Perflexane (USAN, rINN) NH I O perflutren contrast agents for echocardiography.