Mri Contrast Medium and Diagnostic Method
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Caracterización Fisicoquímica Y Clínica De Los Medios De Contraste Intravasculares Iodados
Anales de Radiología México 2008;2:129-140. ARTÍCULOS DE REVISIÓN Dra. Patricia Rodríguez Nava,1 Dr. Ernesto J. Dena Espinoza,1 Caracterización fisicoquímica y Dr. Roberto Basile Lenge,2 Dra. Margarita Fuentes García,3 clínica de los medios de contraste Dr. Gustavo Fink Josephi,4 Dr. Eduardo Marbez Namnum1 intravasculares iodados RESUMEN Objetivo: El propósito de sido aceptado por algunos ra- este artículo es realizar una re- diólogos. La lopromida puede Introducción: El uso de visión bibliográfica sobre las ca- ser considerada un agente uni- medios de contraste intravascu- racterísticas, clasificación, pro- versal para todas las explora- lares no iónicos (gas, sustancia piedades físico-químicas, así ciones y procedimientos radio- hidrosoluble o liposoluble) en como los efectos secundarios lógicos. imagenología ha proliferado (quimiotoxicidad) de los medios en los últimos años debido a su de contraste intravasculares no Palabras clave: Medios de excepcional tolerancia por los iónicos. contraste intravasculares ioda- pacientes. La baja osmolalidad Conclusiones: Un factor que dos, medios de contraste ióni- de este tipo de medios de con- interviene en la incidencia de cos, medios de contraste no ió- traste aporta beneficios como reacciones idiosincráticas o aler- nicos. bajo incremento del volumen goides puede ser el estado psi- sanguíneo, baja toxicidad, bajo cológico del paciente. El trata- efecto sobre la barrera hema- miento previo con antihitamíni- toencefálica. cos, corticosteroides o ambos ha continúa en la pág. 130 1 Del Servicio de Radiología e Imagen “Dr. Carlos Coqui” del Hospital General de México osmolalidad o iso-osmolares. En los Estados Unidos 2 3 O.D. De la Universidad de Buenos Aires Cardiología. -

REGISTRATION DOCUMENT 2017 Sommaire
REGISTRATION DOCUMENT 2017 sommaire Interview with History 4 Yves L’Épine, Chief Executive Officer 2 Key figures 6 1 4 THE GUERBET GROUP 9 MANAGEMENT REPORT 55 1.1 History of the Company 9 4.1 Analysis of the Group’s activity and results 55 1.2 Mission and ambition 10 4.2 Major events since the start of 2018 58 1.3 Main consolidated data 10 4.3 Information about internal control 59 1.4 Overview of activities 11 4.4 Risk management and risk factors 60 1.5 Industrial and logistics activity 17 4.5 Other legal information 64 1.6 Research and Development 18 1.7 The Group’s governance structure at December 31, 2017 22 5 CORPORATE SOCIAL RESPONSIBILITY 69 2 Employee information 70 CORPORATE GOVERNANCE 27 5.1 5.2 Environmental information 76 2.1 Report of the Board of Directors on 5.3 Social information 80 corporate governance 27 5.4 Report by one of the Statutory Auditors, 2.2 Board of Directors 27 designated as an independent third-party, 2.3 General Management 38 on the consolidated human resources, 2.4 Compensation of company officers 38 environmental and social information 2.5 Agreements referred to in Article L. 225- included in the management report 82 37-4-2° of the French Commercial Code 45 2.6 Provisions in the articles of association 6 relating to General Meetings of Shareholders 46 FINANCIAL STATEMENTS 2.7 Deviations from the recommendations for the composition of the Board of AND RELATED NOTES 85 Directors and the Committees 47 6.1 Consolidated financial statements and notes 86 2.8 Other information from the corporate 6.2 Statutory -

A034047-100 Top Secrets.Qxd 5/18/06 2:32 PM Page 1
A034047-100 Top Secrets.qxd 5/18/06 2:32 PM Page 1 TOP 100 SECRETS These secrets are 100 of the top board alerts. They summarize the concepts, principles, and most salient details of CT body scans. 1. Computed tomography (CT) differs from conventional radiography by forming a cross-sectional image, eliminating the superimposition of structures that occurs in plain film imaging because of compression of three-dimensional body structures onto the two-dimensional recording system. 2. The sensitivity of CT to subtle differences in x-ray attenuation is at least a factor of 10 higher than normally achieved by film screen recording systems because of the virtual elimination of scatter. 3. Radiation doses in CT range from 15–50 mGy, depending on exam type. In general, these doses are at least 10 times higher than in radiographic exams. 4. Always seek to optimize scan parameters to minimize patient dose consistent with diagnostic image quality: Minimize mAs, minimize kVp, maximize pitch, maximize slice thickness, and maximize collimator setting. 5. When scanning children, be sure to use pediatric protocols, not adult protocols. In pediatric protocols the CT scan parameters should be reduced so that radiation doses are optimized according to patient size. 6. Pregnant women should be scanned only if there is adequate justification to avoid unnecessary fetal irradiation. 7. Many of the side effects of contrast media are entirely or mainly due to high osmolality. The other causes of side effects due to contrast media are chemotoxicity (allergic-like symptoms), ion toxicity (interference with cellular function), and those caused by a high dose. -

Structure and Properties of X-Ray Contrast Media
STRUCTURE AND PROPERTIES OF X-RAY CONTRAST MEDIA Optimal use of CM in radiology requires a knowl- edge of the nature and relevant properties of the available substances. This chapter describes the properties of currently used and newly developed contrast-giving agents that infuence their behav- ior in the human body, their side effects, and their practical utility. The main X-ray contrast agents in use today are insoluble barium sulfate for the diagnostic evalu- ation of the GI tract and water-soluble CM for the radiological assessment of the different vascular systems, body cavities and organs. In addition, a water-soluble CM based on tri-iodobenzene is the alternative agent of choice for oral use when bari- um sulfate is contraindicated. Barium Sulfate Barium is used in the form of the insoluble sulfate for radiography of the GI tract. If perforation is suspected, however, only water-soluble, iodinated agents (Gastrografn, Ultravist-370) can be used since the body is virtually incapable of eliminating barium sulfate once it has entered the peritoneum. Barium sulfate is available either as a powder to be prepared directly before use or as a ready-to-use suspension. For double-contrast examinations (fll- ing of the lumen with gas, coating of the wall with barium sulfate), barium sulfate is either mixed with a carbon dioxide additive, or a gas-forming agent is taken in addition. © The Author(s) 2018 20 U. Speck, X-Ray Contrast Media, https://doi.org/10.1007/978-3-662-56465-3_3 Common to all barium preparations is concentra- tion of barium sulfate which may diluted according to the needs of the examination. -
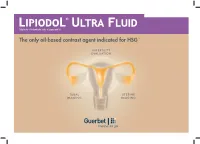
The Only Oil-Based Contrast Agent Indicated for HSG1-9
Ethyl ester of iodized fatty acids of poppy seed oil The only oil-based contrast agent indicated for HSG 1-9 INFERTILITY EVALUATION TUBAL UTERINE IMAGING IMAGING FOR HYSTEROSALPINGOGRAPHY Pharmaceutical form: Lipiodol® Ultra Fluid 480 mg Iodine per mL, solution for injection 10 mL - ethyl esters of iodized fatty acids of poppy-seed oil Posology/Recommended dosage: Up to 20 mL, depending on the volume of the uterine cavity 2-3 P 4 Lipiodol® Ultra Fluid for Hysterosalpingography (HSG) P 5 Reasons for performing HSG P 6 The female reproductive system P 7 When to perform HSG P 8 HSG steps P 9 Lipiodol® HSG: Findings P 10 Lipiodol® HSG: Endorsement by international clinical practice guidelines P 11 Lipiodol® HSG: Safety P 12 Lipiodol® HSG: Pain P 13 Lipiodol® HSG: Features & Benefits P 14 Bibliography Lipiodol® Ultra Fluid for Hysterosalpingography ( ) Hysterosalpingography: radiological examination to investigate the uterine cavity, Fallopian tubes & peritoneal cavity. It entails the injection of contrast medium and visualization under fluoroscopy. 10 CHARACTERIZATION OF HSG FINDINGS: Tubal abnormalities Uterine cavity abnormalities Tubal occlusion Congenital anomalies Salpingitis isthmica nodosum Polyps Polyps Leiomyomas Hydrosalpinx Surgical changes Peritubal adhesions Synechiae Adenomyosis Müllerian duct anomalies Source: Simpson WL Jr et al. Hysterosalpingography: a reemerging study. Radiographics. 2006 Mar-Apr;26(2):419-31. - Simple & accurate procedure for tubal patency & uterine investigation 4-5 Lipiodol® Ultra Fluid for Hysterosalpingography -

WO 2017/142292 Al
/OA (12) 특허협력 조약에 의하여 공개된 국제출원 (19) 세계지 식재산권기구 국제사무국 (10) 국제공개번호 (43) 국제공개일 WO 2017/142292 A l 2017 년 8 월 24 일 (24.08.2017) W Ο P C T (51) 국제특허 분류: Incheon (KR). 최병현 (CHOI, Byeong-Hyeon); 08308 서 0 67/(927 (2006.01) G01N 33/50 (2006.01) 울 시 구로구 구로동로 148, 연구동 2 층 흉부 외과 연구 실, Seoul (KR). (21) 국제출원번호: PCT/KR2017/001610 (74) 대 리 인 : 특 허 법 인 충 현 (CHUNG HYUN PATENT (22) 국제출원일: 2017 년 2 월 14 일 (14.02.2017) LAW FIRM); 06779 서울 시 서초 구 동산로 23 베 델 회 (25) 출원언어 : 한국어 관 8 층, Seoul (KR). (26) 공개언어 : (81) 지정국 ( 별도 의 표 시가 없는 한 , 가능한 모든 종류의 국 내 권 리 의 보 호 를 위 하 여 ) : AE, AG, AL, AM, AO, (30) 우선권정보: AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 10-2016-0017226 2016 년 2 월 15 일 (15.02.2016) K R CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, 년 월 일 10-2017-00193 12 2017 2 13 ^13.02.2017; K R DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (71) 출원인: 고 려대학교 산학협력단 (KOREA UNIVER HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KH, KN, SITY RESEARCH AND BUSINESS FOUNDATION) KP, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, [KR/KR]; 0284 1 서 울 시 성 북 구 안 암 로 145, Seoul ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, CM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (KR). -

Appendix B - Product Name Sorted by Applicant
JUNE 2021 - APPROVED DRUG PRODUCT LIST B - 1 APPENDIX B - PRODUCT NAME SORTED BY APPLICANT ** 3 ** 3D IMAGING DRUG * 3D IMAGING DRUG DESIGN AND DEVELOPMENT LLC AMMONIA N 13, AMMONIA N-13 FLUDEOXYGLUCOSE F18, FLUDEOXYGLUCOSE F-18 SODIUM FLUORIDE F-18, SODIUM FLUORIDE F-18 3M * 3M CO PERIDEX, CHLORHEXIDINE GLUCONATE * 3M HEALTH CARE INC AVAGARD, ALCOHOL (OTC) DURAPREP, IODINE POVACRYLEX (OTC) 3M HEALTH CARE * 3M HEALTH CARE INFECTION PREVENTION DIV SOLUPREP, CHLORHEXIDINE GLUCONATE (OTC) ** 6 ** 60 DEGREES PHARMS * 60 DEGREES PHARMACEUTICALS LLC ARAKODA, TAFENOQUINE SUCCINATE ** A ** AAA USA INC * ADVANCED ACCELERATOR APPLICATIONS USA INC LUTATHERA, LUTETIUM DOTATATE LU-177 NETSPOT, GALLIUM DOTATATE GA-68 AAIPHARMA LLC * AAIPHARMA LLC AZASAN, AZATHIOPRINE ABBVIE * ABBVIE INC ANDROGEL, TESTOSTERONE CYCLOSPORINE, CYCLOSPORINE DEPAKOTE ER, DIVALPROEX SODIUM DEPAKOTE, DIVALPROEX SODIUM GENGRAF, CYCLOSPORINE K-TAB, POTASSIUM CHLORIDE KALETRA, LOPINAVIR NIASPAN, NIACIN NIMBEX PRESERVATIVE FREE, CISATRACURIUM BESYLATE NIMBEX, CISATRACURIUM BESYLATE NORVIR, RITONAVIR SYNTHROID, LEVOTHYROXINE SODIUM ** TARKA, TRANDOLAPRIL TRICOR, FENOFIBRATE TRILIPIX, CHOLINE FENOFIBRATE ULTANE, SEVOFLURANE ZEMPLAR, PARICALCITOL ABBVIE ENDOCRINE * ABBVIE ENDOCRINE INC LUPANETA PACK, LEUPROLIDE ACETATE ABBVIE ENDOCRINE INC * ABBVIE ENDOCRINE INC LUPRON DEPOT, LEUPROLIDE ACETATE LUPRON DEPOT-PED KIT, LEUPROLIDE ACETATE ABBVIE INC * ABBVIE INC DUOPA, CARBIDOPA MAVYRET, GLECAPREVIR NORVIR, RITONAVIR ORIAHNN (COPACKAGED), ELAGOLIX SODIUM,ESTRADIOL,NORETHINDRONE ACETATE -

Super-Selective Transarterial Chemoembolization With
Balli, H, et al. Super-Selective Transarterial Chemoembolization with Doxorubicin-Loaded Drug-Eluting Beads Sized Below and Above 100 Microns in Hepatocellular Carcinoma: A Comparative Study. Journal of the Belgian Society of Radiology. 2019; 103(1): 47, 1–7. DOI: https://doi.org/10.5334/jbsr.1841 ORIGINAL ARTICLE Super-Selective Transarterial Chemoembolization with Doxorubicin-Loaded Drug-Eluting Beads Sized Below and Above 100 Microns in Hepatocellular Carcinoma: A Comparative Study Huseyin Balli, Erol Aksungur, Behruz Khalatai and Kairgeldy Aikimbaev Objectives To compare efficacy and safety of super-selective DEB-TACE with doxorubicin-loaded micro- spheres sized below and above 100 microns for treatment of hepatocellular carcinoma (HCC). Material and methods All consecutive patients with HCC who underwent DEB-TACE were included in this retrospective study. Regarding to microsphere size (>100 microns or <100 microns), patients were determined as Group A (n = 28) and Group B (n = 30), respectively. Results Of the 58 patients (78% males), no statistically sig- nificant difference was found between the two groups in terms of age and gender (P = 0.388, P = 0.888, respectively). There were no significant differences between the two groups in terms of BCLC stages, presence of chronic liver disease, and Child-Pugh classes (P = 0.593, P = 0.081, P = 0.391, respectively). Although statistically insignificant, median overall survival (19 months vs 32 months, P = 0.190) and median progression-free survival (13 months vs 20 months (P = 0.574) were longer and 1-3-years objec- tive response rates (7.40% vs 23.33%, P = 0.330) were higher in Group B than in Group A, respectively. -

Produits Iodes
COMMISSION DE LA TRANSPARENCE Avis 15 mai 2013 REEVALUATION DES PRODUITS DE CONTRASTE IODES Code ATC (2012) V08A (produits de contraste iodés) Réévaluation du service médical rendu à la demande de la Commission, Motif de l’examen (conformément à l’article R 163-21 du CSS) Sécurité Sociale (CSS L.162-17) Listes concernées Collectivités (CSP L.5123-2) Annexe – réévaluation des produits de contraste iodés – Synthèse-Avis 3 1/14 01 INFORMATIONS ADMINISTRATIVES ET REGLEMENTAIRES Conditions de prescription et de Liste I- Médicaments soumis à prescription médicale délivrance 02 CONTEXTE Les spécialités radiodiagnostiques iodées inscrites sur la liste des spécialités remboursables aux assurés sociaux sont examinées dans le cadre de renouvellement d’inscription et d’une réévaluation du service médical rendu à l’initiative de la Commission de la transparence, suite à l’alerte émise par le groupe de travail de l’ANSM dans le cadre de l’initialisation d’une réévaluation démarrée fin 2011 (cf. Synthèse : Réévaluation des produits de contraste iodés). Le groupe des produits de contraste iodés (PCI) comprend les produits de basse osmolalité et les produits de haute osmolalité. La présente réévaluation concerne plus particulièrement la place des produits de contraste de haute osmolalité, hydrosolubles, administrés par voie vasculaire. Les produits de contraste iodés sont constitués de 3 atomes d’iode fixés sur 1 ou 2 cycles benzéniques. On distingue aujourd’hui 2 types de PCI en fonction de leur osmolalité : - Les PCI dits classiques ou PCI de haute osmolalité sont des monomères (1 seul cycle benzénique) ioniques, c’est-à dire associés à un cation sodium ou méglumine. -

(12) Patent Application Publication (10) Pub. No.: US 2012/0184642 A1 Bartling Et Al
US 2012O184642A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2012/0184642 A1 Bartling et al. (43) Pub. Date: Jul.19, 2012 (54) MULTIMODAL VISIBLE POLYMER (30) Foreign Application Priority Data EMBOLIZATION MATERAL Jul. 7, 2009 (DE) ......................... 102O09.0321.89.6 (76) Inventors: Soenke Bartling, Heidelberg (DE); O O Shlomo Margel, Rehovot (IL); Publication Classification Hagit Aviv, Givart Shmuel (IL) (51) Int. Cl. A6IL 3L/04 (2006.01) (21) Appl. No.: 13/382,352 A6IL 3L/06 (2006.01) A6IL 3 L/18 (2006.01) (22) PCT Filed: Jul. 6, 2010 (52) U.S. Cl. ........................................................ 523/113 (57) ABSTRACT (86). PCT No.: PCT/EP2010/059631 The present invention relates to embolization material for S371 (c)(1), therapeutic use, wherein said material is visible via more than (2), (4) Date: Mar. 23, 2012 one imaging technique. Patent Application Publication Jul. 19, 2012 Sheet 1 of 19 US 2012/O184642 A1 8 3 x 8888: 83 x 38 : Patent Application Publication Jul. 19, 2012 Sheet 2 of 19 US 2012/O184642 A1 Patent Application Publication Jul. 19, 2012 Sheet 3 of 19 US 2012/O184642 A1 & S S S S S SS &S & S& SS S S & S S Patent Application Publication Jul. 19, 2012 Sheet 4 of 19 US 2012/O184642 A1 Patent Application Publication Jul. 19, 2012 Sheet 5 of 19 US 2012/O184642 A1 38 : x3.3 8:::::::::::::::: Patent Application Publication Jul.19, 2012 Sheet 6 of 19 US 2012/0184642 A1 Patent Application Publication Jul. 19, 2012 Sheet 7 of 19 US 2012/O184642 A1 Patent Application Publication Jul. -

CIN) and Its Link to in Vitro Studies on Iodinated Contrast Media (CM
BioMedicine (ISSN 2211-8039) DOI: 10.1051/bmdcn/2018080101 March 2018, Vol. 8, No. 1, Article 1, Pages 1-11 Review article The molecular mechanism of contrast-induced nephropathy (CIN) and its link to in vitro studies on iodinated contrast media (CM) Jai-Sing Yang1, †, Yan-Ru Peng2, †, Shih-Chang Tsai3, Yeu-Sheng Tyan4, 5, 6, Chi-Cheng Lu7, Hong-Yi Chiu7, Yu-Jen Chiu8, Sheng-Chu Kuo9, 10, Yuh-Feng Tsai11, 12, Ping-Chin Lin13, Fuu-Jen Tsai14, 15, 16,* 1Department of Medical Research, China Medical University Hospital, China Medical University, Taichung 404, Taiwan 2School of Medicine, China Medical University, Taichung 404, Taiwan 3Department of Biological Science and Technology, China Medical University, Taichung 404, Taiwan 4Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung 402, Taiwan 5School of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung 402, Taiwan 6School of Medicine, Chung Shan Medical University, Taichung 402, Taiwan 7Department of Pharmacy, Buddhist Tzu Chi General Hospital, Hualien 970, Taiwan. 8Division of Reconstructive and Plastic Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei 112, Taiwan. 9Chinese Medicinal Research and Development Center, China Medical University Hospital, China Medical University, Taichung 404, Taiwan 10School of Pharmacy, China Medical University, Taichung 404, Taiwan 11Department of Diagnostic Radiology, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei 111, Taiwan 12School of Medicine, Fu-Jen Catholic University, Taipei 242, Taiwan 13Department of Medical Imaging, Chia-Yi Christian Hospital, Chiayi 600, Taiwan 14Genetics Center, Department of Medical Research, China Medical University Hospital, Taichung 404, Taiwan 15School of Chinese Medicine, China Medical University, Taichung 404, Taiwan 16Department of Medical Genetics, China Medical University Hospital, Taichung 404, Taiwan Received 30th of October, 2017 Accepted 7th of November, 2017 © Author(s) 2018. -
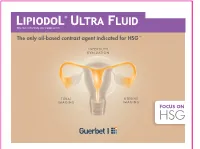
The Only Oil-Based Contrast Agent Indicated for HSG1-9
Ethyl ester of iodized fatty acids of poppy seed oil The only oil-based contrast agent indicated for HSG 1-9 INFERTILITY EVALUATION TUBAL UTERINE IMAGING IMAGING FOCUS ON HSG Hysterosalpingography ( ) def inition HSG definition HSG: examination evaluating the uterine cavity & Fallopian tubes. It entails the injection of contrast medium and visualization with radiographic imaging. 10 HYSTERO SALPINGO UTERUS GRAPHY FALLOPIAN TUBES EXPLORATION The female reproductive system WHEN TO PERFORM HSG? 11 Fallopian tubes: transport sperm after bleeding period and towards the egg for fertilization and HSG must be performed th then fertilized egg to the uterus before ovulation (ideally before the 12 day of the menstrual cycle for women with normal cycle length) system Female reproductive Female reproductive Endometrium Ovaries: secrete estrogen & progesterone; produce Uterus: where foetus develops eggs during pregnancy Cervix Vagina: where semen enters the body; sperm can then travel up to the uterus and into the Fallopian tubes Gonadotropin Hormones: FSH (Follicle Stimulating Hormone) & LH (Luteinizing Hormone) Ovarian Hormones: Estrogen & Progesterone steps PREPARATION The patient is positioned on her back on a table under a fluoroscope, bringing legs up into gynecological position Cervix must be cleaned with an antiseptic Vaginal speculum gently inserted for visualization of cervix Fallopian tube HSG PROCEDURE HSG steps Ovary Uterus Cervix 1 Catheter inserted through the opening in the cervix into the uterus Vagina 2 Contrast medium (Lipiodol® Ultra