Low-Molecular-Weight Thiols: Identification of Novel Thiol Compounds and Applications in Winemaking Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
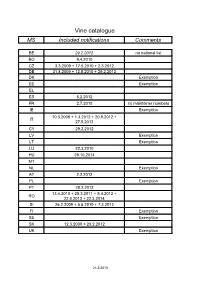
Vine Catalogue MS Included Notifications Comments
Vine catalogue MS Included notifications Comments BE 29.2.2012 no national list BG 9.4.2010 CZ 3.3.2009 + 17.5.2010 + 2.3.2012 DE 31.8.2009 + 12.5.2010 + 29.2.2012 DK Exemption EE Exemption EL ES 8.2.2012 FR 2.7.2010 no maintainer numbers IE Exemption 10.3.2008 + 1.3.2012 + 20.9.2012 + IT 27.5.2013 CY 29.2.2012 LV Exemption LT Exemption LU 22.3.2010 HU 29.10.2014 MT NL Exemption AT 2.2.2012 PL Exemption PT 28.2.2012 13.4.2010 + 25.3.2011 + 5.4.2012 + RO 22.4.2013 + 22.3.2014 SI 26.2.2009 + 5.5.2010 + 7.3.2012 FI Exemption SE Exemption SK 12.3.2009 + 20.2.2012 UK Exemption 21.5.2015 Common catalogue of varieties of vine 1 2 3 4 5 Known synonyms Variety Clone Maintainer Observations in other MS A Abbuoto N. IT 1 B, wine, pas de Abondant B FR matériel certifiable Abouriou B FR B, wine 603, 604 FR B, wine Abrusco N. IT 15 Accent 1 Gm DE 771 N Acolon CZ 1160 N We 725 DE 765 B, table, pas de Admirable de Courtiller B FR matériel certifiable Afuz Ali = Regina Agiorgitiko CY 163 wine, black Aglianico del vulture N. I – VCR 11, I – VCR 14 IT 2 I - Unimi-vitis-AGV VV401, I - Unimi-vitis- IT 33 AGV VV404 I – VCR 7, I – VCR 2, I – Glianica, Glianico, Aglianico N. VCR 13, I – VCR 23, I – IT 2 wine VCR 111, I – VCR 106, I Ellanico, Ellenico – VCR 109, I – VCR 103 I - AV 02, I - AV 05, I - AV 09, I - BN 2.09.014, IT 31 wine I - BN 2.09.025 I - Unimi-vitis-AGT VV411, I - Unimi-vitis- IT 33 wine AGTB VV421 I - Ampelos TEA 22, I - IT 60 wine Ampelos TEA 23 I - CRSA - Regione Puglia D382, I - CRSA - IT 66 wine Regione Puglia D386 Aglianicone N. -

European Project Grapegen 06 - Grapevine Genetic Resources - Version 21 January 2011 P
European Project GrapeGen 06 - Grapevine Genetic Resources European Grapevine Catalogue: Towards a Comprehensive List T. Lacombe, L. Audeguin, M. Boselli, B. Bucchetti, F. Cabello, M. Crespan, C. D’Onofrio, J. Eiras Dias, S. Ercisli, M. Gardiman, MS. Grando, S. Imazio, O. Jandurova, A. Jung, E. Kiss, P. Kozma, E. Maul, D. Maghradze, C. Martinez, G. Muñoz, J-K. Pátková, I. Pejic, E. Peterlunger, D. Pitsoli, D. Preiner, S. Raimondi, F. Regner, G. Savin, S. Savvides, A. Schneider, J-L. Spring, A. Szoke, A. Veres, J-M. Boursiquot, R. Bacilieri and P. This Annex 3 B : Official national catalogues of grapevine varieties for Member States of the European Union and the Third Countries partner of the GrapeGen 06 Project Legend : before the arrows, name of the variety as registered in the country . After the arrows, common prime name of the variety according to VIVC database when referenced, # identification number of the variety, species of the variety, sex (H = hermaphrodite, F = female, M = male), colour of berry skin (B = yellow-green, N = blue-black, Rg = red, Rs = rose, G = grey). Austria AUT National Catalogue version 2008 Alphonse-Lavalle (AUT) >>> ALPHONSE LAVALLEE # 349 - vinifera - H - N Angela (AUT) >>> ANGELA # 20342 - interspecific cross - H - B Aron (AUT) >>> ARON # 14014 - interspecific cross - - B Attica (AUT) >>> ATTIKA SEEDLESS # 17309 - vinifera - - Rg Attila (AUT) >>> ATTILA # 756 - vinifera - - B Bacchus (AUT) >>> BACCHUS WEISS # 851 - - H - B Bianca (AUT) >>> BIANCA # 1321 - interspecific cross - H - B Birstaler Muskat (AUT) -

Evaluation of the CAP Measures Applicable to the Wine Sector
Evaluation of the CAP measures applicable to the wine sector Case study report: Germany – Rhineland-Palatinate Written by Agrosynergie EEIG Agrosynergie November – 2018 Groupement Européen d’Intérêt Economique AGRICULTURE AND RURAL DEVELOPMENT EUROPEAN COMMISSION Directorate-General for Agriculture and Rural Development Directorate C – Strategy, simplification and policy analysis Unit C.4 – Monitoring and Evaluation E-mail: [email protected] European Commission B-1049 Brussels EUROPEAN COMMISSION Evaluation of the CAP measures applicable to the wine sector Case study report: Germany – Rhineland-Palatinate Directorate-General for Agriculture and Rural Development 2018 EN Europe Direct is a service to help you find answers to your questions about the European Union. Freephone number (*): 00 800 6 7 8 9 10 11 (*) The information given is free, as are most calls (though some operators, phone boxes or hotels may charge you). LEGAL NOTICE The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the Commission. The Commission does not guarantee the accuracy of the data included in this study. Neither the Commission nor any person acting on the Commission’s behalf may be held responsible for the use which may be made of the information contained therein. More information on the European Union is available on the Internet (http://www.europa.eu). Luxembourg: Publications Office of the European Union, 2019 Catalogue number: KF-04-18-977-EN-N ISBN: 978-92-79-97270-6 doi: 10.2762/09274 © European Union, 2018 Reproduction is authorised provided the source is acknowledged. -

Existing Wine Names - Technical File
TECHNICAL FILE 1 /12 File number: PDO-DE-A1272 Existing wine names - Technical file I. NAME(S) TO BE REGISTERED Pfalz (de) II. APPLICANT DETAILS Applicant name and title Rhineland-Palatinate Ministry of the Environment, Agriculture, Food, Viticulture and Forestry Legal status, size and Regional authority under public law composition (in the case of legal persons) Nationality Germany Address 1 Kaiser-Friedrich-Str. 55116 Mainz Germany Tel.: 0049-06131 - 16 - 0 Fax: 0049-06131 - 16 - 4646 E-mail(s): [email protected] III. PRODUCT SPECIFICATION Status: Enclosed File name gU Pfalz_111219.pdf IV. NATIONAL DECISION OF APPROVAL Legal basis The national decision of approval for ‘Pfalz’ was issued under the Wine Legislation Reform Act of 8 July 1994 (BGBl I, p. 1467). V. SINGLE DOCUMENT Name(s) to be registered Pfalz (de) Equivalent term(s): TECHNICAL FILE 2 /12 File number: PDO-DE-A1272 Traditionally used name: No Legal basis for the Article 118s of Regulation (EC) No 1234/2007 transmission: The present technical file includes amendments(s) adopted according to: Geographical indication PDO - Protected Designation of Origin type: 1. CATEGORIES OF GRAPEVINE PRODUCTS 1. Wine 5. Quality sparkling wine 8. Semi-sparkling wine 2. DESCRIPTION OF THE WINE(S) Analytical characteristics: Description of the wine(s) 2.1. Analytical The analysis values listed below, which must be determined by means of a physical and chemical analysis in accordance with Article 26 of Regulation (EC) No 607/2009, are binding minimum values which must be present in the given wine varieties for use of the designation to be allowed: • Not less than 5.5 % actual alcoholic strength by volume for Beerenauslese etc., or 7 % actual alcoholic strength by volume for quality wine. -

Sonderheft 2015
48. Jahrgang Hannover, den 15. April 2015 Seite 1 - 117 SONDERHEFT SORTENREGISTER Stand vom 1. April 2015 A Nach dem SaatG zugelassene Sorten, Sorten mit Auslauffristen und Sorten nach 2 § 55 Abs. 2 SaatG sowie Hinweise nach § 33 Abs. 8 SaatgutV 1. Nach dem SaatG zugelassene Sorten 2 2. Sorten mit Auslauffristen 32 3. Inhalt der Auflagen 33 4. Sorten nach § 55 SaatG, von denen Saatgut anerkannt werden kann 33 5. Obstsorten, die nach § 6 Abs. 4 der Verordnung über das Inverkehrbringen 81 von Anbaumaterial von Gemüse-, Obst- und Zierpflanzenarten (Anbaumaterialverordnung) anerkannt werden können 6. Hinweis auf die Erhaltungszüchtung nach § 33 Abs. 8 SaatgutV 89 B Nach dem SortG geschützte Sorten, die nicht zugelassen sind 90 C Verzeichnis 104 der Sortenschutzinhaber, Züchter, weiteren Züchter, Verfahrensvertreter, Bevollmächtigten und Nutzungsberechtigten Seite 2 Blatt für Sortenwesen 2015 SORTENREGISTER Stand 1. April 2015 Die eingeklammerten Zahlen hinter den Sortenbezeichnungen verweisen auf die lfd. Nummer des Anschriftenverzeichnisses. Sie bedeuten: Ohne Buchstabe: Sortenschutzinhaber/Züchter/weiterer Züchter V: Verfahrensvertreter nach § 15 Abs. 2 SortG/§ 42 Abs. 6 Saat B: Bevollmächtigter N: Nutzungsberechtigter nach § 11 Abs. 2 SortG C vor der Kennummer bedeutet: Codebezeichnung Hinter jeder Sorte ist die Kennummer angegeben, unter der die Sorte beim Bundessortenamt geführt wird. Bei Rebsorten werden außerdem nach der Sortenbezeichnung und der Nummer im Anschriftenverzeichnis, die zugehörigen Klone angegeben. A Nach dem SaatG zugelassene Sorten, Sorten mit Auslauffristen und Sorten nach § 55 Abs. 2 SaatG sowie Hinweise nach § 33 Abs. 8 SaatgutV Sorten, die erstmals bzw. erstmals wieder aufgeführt werden, sind durch ein + vor der Sortenbezeichnung kenntlich gemacht. Hinter den Kennummern sind in Klammern für die Anerkennung von Saatgut relevante Hinweise aufgeführt. -

Gga Landwein Rhein 111215
Ministerium für Klimaschutz, Umwelt, Landwirtschaft, Natur- und Verbraucherschutz des Landes Nordrhein-Westfalen Hessisches Ministerium für Umwelt, Energie, Landwirtschaft und Verbraucherschutz „Landwein Rhein“ Produktspezifikation für eine geschützte geografische Angabe … Produktspezifikation für eine geschützte geografische Angabe „Landwein Rhein“ 1. Geschützter Name „Landwein Rhein“ Die geschützte geografische Angabe (g.g.A.) „Landwein Rhein“ erstreckt sich auf Land- weine aus den Ländern Rheinland-Pfalz, Nordrhein-Westfalen und Hessen. 2. Beschreibung des Weines 2.1. Analytisch Nachfolgend aufgeführte Analysewerte, die anhand einer physikalischen und chemi- schen Analyse gemäß Artikel 26 der Verordnung (EG) Nr. 607/2009 zu ermitteln sind, sind verbindlich vorgegebene Mindest- und Höchstwerte, die bei Landwein eingehalten werden müssen, um die Bezeichnung verwenden zu dürfen: • Vorhandener Alkoholgehalt von mindestens 4,5 % vol. • Gesamtalkoholgehalt nach Anreicherung max. 11,5 % vol bei Weiß- und Roséwein sowie max. 12 %vol bei Rotwein. • Entsprechend dem Gesamtzuckergehalt dürfen folgende Geschmacksangaben ver- wendet werden: Geschmacksangabe Zuckergehalt: trocken Wenn der Zuckergehalt folgende Werte nicht über- schreitet: - 4 g/l oder - 9 g/l, sofern der in g/l Weinsäure ausgedrückte Ge- samtsäuregehalt höchstens um 2 g/l niedriger ist als der Restzuckergehalt. halbtrocken Wenn der Zuckergehalt den vorgenannten Höchstwert überschreitet, folgende Werte aber nicht überschreitet: - 12 g/l oder - 18 g/l, sofern der in g/Liter Weinsäure ausgedrückte Gesamtsäuregehalt höchstens um 10 g/l niedriger ist als der Restzuckergehalt. lieblich Wenn sein Zuckergehalt den vorgenannten Höchst- wert überschreitet, aber nicht mehr als 45 g/l beträgt. süß Wenn sein Zuckergehalt mindestens 45 g/l beträgt. • Gesamtsäure muss mindestens 3,5 g/l betragen. • Gehalte an flüchtige Säure (Maximalwerte): a) 18 Milliäquivalent je Liter bei Weißwein und Roséwein, b) 20 Milliäquivalent je Liter bei Rotwein. -

German White Wines – Steve Zins 11/08/2017 Rev 3.0
German White Wines – Steve Zins 11/08/2017 Rev 3.0 Contents • Introduction • German Wine - fun facts • German Geography • Wine Regions • Wine Production • Trends • Permitted Whites • Label Markings • Wine Classification Old and New VDP • Wine Tasting • Conclusion • References Introduction • Seems like yesterday I presented German Red wines. As a matter of fact it was 11/12/2014! • We visit Germany at least once a year. We always try to visit some new regions and vineyards. • I am always surprised how many good wines are available. Generally they are all reasonably priced. • I sourced all the wines we will taste from Surdyks in Minneapolis. Great luck for the club, the fall sale was on and all wines were 20% off. German Wine - fun facts • 90% of German wines are consumed in Germany. • Very few wine retailers in America have a good selection of German wines. • Most of the largest white producers are still too small to export to USA. • Many cooperatives blend and ship Liebfraumilch , Gewürztraminer , and some Riesling on the low end of the market. • As vineyard owners die the vineyards are split between siblings. Some vineyards get down to 3 rows. Siblings take turns picking the center row from year to year. • High quality German Riesling does not come in a BLUE BOTTLE ! • I don’t recall ever seeing a BLUE BOTTLE while in Germany. German Geography • Germany is 138,000 sq mi or 357,000 sq km • Germany is approximately the size of Montana ( 146,000 sq mi ) • Germany is divided with respect to wine production into the following: • 13 Regions -

Rheingauer Landwein
Hessisches Ministerium für Umwelt, Energie, Landwirtschaft und Verbraucherschutz Rheingauer Landwein Produktspezifikation für eine geschützte geographische An- gabe 1. Geschützter Name „Rheingauer Landwein“ ____ 2. Beschreibung des Weines/der Weine Im beschriebenen Landweingebiet werden insbesondere Weißweine und daneben auch traditionell Rot- und Roséweine hergestellt. Stand 31. Juli 2010 waren von der gesamten Fläche rd. 85 % mit weißen Rebsorten und rd. 15 % mit roten Rebsorten bepflanzt. Dabei liegen die Schwerpunkte auf den Rebsorten Riesling (78,9 % der Rebfläche) und Spätburgunder (12,2 % der Rebfläche). ____ 2.1 Sensorisch Die Weine spiegeln ihren Standort mit seinen geologischen, morphologischen und natür- lichen Einflüssen (siehe 3.1 und 3.2) sowie die Arbeit des Winzers in Weinberg und Kel- ler wider. Weißweine zeigen hellgrüne bis gelbliche Farbtöne und weisen oft Pfirsich-, Aprikosen- oder Zitrusaromen auf. Rotweine sind von hell- bis dunkelroter Farbe und werden durch Kirscharomen oder die Aromen dunkler Beerenfrüchte geprägt. Weiß- herbste und Roséweine sind von heller bis blassroter Farbe, ihre Aromen erinnern meist an rötliche Beerenfrüchte. Je nach Standort und Rebsorte sind die aromatischen und mineralischen Komponenten unterschiedlich stark ausgeprägt. Die Weine werden in den Geschmacksrichtungen trocken oder halbtrocken ausgebaut, sie weisen aber nicht die ____ Fülle und den Alkoholgehalt von Qualitäts- und Prädikatsweinen auf. 2.2 Analytisch Nachfolgend aufgeführte Analysewerte, die anhand einer physikalischen und chemi- schen Analyse gemäß Artikel 26 der VO (EG) Nr. 607/2009 zu ermitteln sind, sind ver- bindlich vorgegeben und müssen eingehalten werden, um die Bezeichnung „Rheingauer Landwein“ verwenden zu dürfen: • Gesamtalkoholgehalt nach Anreicherung max. 11,5 % vol. bei Weißwein • Gesamtalkoholgehalt nach Anreicherung max. 12,0 % vol. -

Annex 3A V3-4
European Project GrapeGen 06 - Grapevine Genetic Resources European Grapevine Catalogue: Towards a Comprehensive List T. Lacombe, L. Audeguin, M. Boselli, B. Bucchetti, F. Cabello, M. Crespan, C. D’Onofrio, J. Eiras Dias, S. Ercisli, M. Gardiman, MS. Grando, S. Imazio, O. Jandurova, A. Jung, E. Kiss, P. Kozma, E. Maul, D. Maghradze, C. Martinez, G. Muñoz, J-K. Pátková, I. Pejic, E. Peterlunger, D. Pitsoli, D. Preiner, S. Raimondi, F. Regner, G. Savin, S. Savvides, A. Schneider, J-L. Spring, A. Szoke, A. Veres, J-M. Boursiquot, R. Bacilieri and P. This Annex 3 A : Official national catalogues of grape varieties for Member States of the European Union Legend : before the arrows, name of the variety as registered in the country . After the arrows, common prime name of the variety according to VIVC database when referenced, # identification number of the variety, species of the variety, sex (H = hermaphrodite, F = female, M = male), colour of berry skin (B = yellow-green, N = blue-black, Rg = red, Rs = rose, G = grey). Austria AUT National Catalogue version 2008 Alphonse-Lavalle (AUT) >>> ALPHONSE LAVALLEE # 349 - vinifera - H - N Angela (AUT) >>> ANGELA # 20342 - interspecific cross - H - B Aron (AUT) >>> ARON # 14014 - interspecific cross - - B Attica (AUT) >>> ATTIKA SEEDLESS # 17309 - vinifera - - Rg Attila (AUT) >>> ATTILA # 756 - vinifera - - B Bacchus (AUT) >>> BACCHUS WEISS # 851 - - H - B Bianca (AUT) >>> BIANCA # 1321 - interspecific cross - H - B Birstaler Muskat (AUT) >>> BIRSTALER MUSKAT # 17208 - interspecific cross - H - B Blauburger -

Related Titles
Related titles Winemaking problems solved (ISBN 978-1-84569-475-3) Managing wine quality Volume 1: Viticulture and wine quality (ISBN 978-1-84569-484-5) Managing wine quality Volume 2: Oenology and wine quality, (ISBN 978-1-84569-798-3) Woodhead Publishing Series in Food Science, Technology and Nutrition: Number 268 Grapevine Breeding Programs for the Wine Industry Edited by Andrew Reynolds AMSTERDAM • BOSTON • CAMBRIDGE • HEIDELBERG LONDON • NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Woodhead Publishing is an imprint of Elsevier Woodhead Publishing is an imprint of Elsevier 80 High Street, Sawston, Cambridge, CB22 3HJ, UK 225 Wyman Street, Waltham, MA 02451, USA Langford Lane, Kidlington, OX5 1GB, UK Copyright © 2015 Elsevier Ltd. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publisher. Permissions may be sought directly from Elsevier’s Science & Technology Rights Department in Oxford, UK: phone (+44) (0) 1865 843830; fax (+44) (0) 1865 853333; email: [email protected]. Alternatively, you can submit your request online by visiting the Elsevier website at http://elsevier.com/locate/permissions, and selecting Obtaining permission to use Elsevier material. Notice No responsibility is assumed by the publisher for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions or ideas contained in the material herein. Because of rapid advances in the medical sciences, in particular, independent verification of diagnoses and drug dosages should be made. -

Beschreibende Sortenliste Reben 2015
Beschreibende Sortenliste Reben 2015 Bundessortenamt 2015 Die vom Herausgeber gewählte Aufmachung der Broschüre darf ohne Genehmigung nicht verändert werden. Nachdruck nur mit Quellenangabe gestattet. Herausgeber: Bundessortenamt, Osterfelddamm 80, 30627 Hannover Bezug durch: Bundessortenamt Osterfelddamm 80, 30627 Hannover Telefon-Nr.: (0511) 9566-5732 Fax-Nr.: (0511) 9566-9600 Internet: www.bundessortenamt.de E-Mail: [email protected] ISSN 14 30 - 93 86 3 Vorwort Die Wahl der Rebsorte ist nicht zuletzt angesichts des Sortenbewusstseins des Endver- brauchers eine der wichtigsten Entscheidungen eines Betriebes, da sie in erheblichem Maße dessen Wirtschaftlichkeit mitbestimmt. Das Bundessortenamt erstellt daher entsprechend den Vorgaben des Saatgutverkehrsgesetzes eine Beschreibende Sortenliste für Reben, um von neutraler Seite Informationen zu den physiologischen Merkmalen, insbesondere den Anbaueigenschaften und dem Verwendungszweck zu geben und so die Sortenwahl zu unterstützen. Die vorliegende Beschreibende Sortenliste Reben enthält umfassende Angaben zu allen in Deutschland nach dem Stand vom 01. Januar 2015 saatgutrechtlich zugelassenen 112 Ertragsrebsorten und 16 Unterlagsrebsorten. Sie erscheint in mehrjährigen Abstän- den. Die letzte Ausgabe stammt aus dem Jahr 2008. Kurzbeschreibungen der zwischen- zeitlich neu zugelassenen Sorten sowie die aktuellen Verfahrensstände der Sorten sind jeweils unter www.bundessortenamt.de abrufbar. Eine Gesamtübersicht aller zugelas- senen und geschützten Sorten sowie ihrer Klone wird alljährlich mit Stand vom 01. April im Blatt für Sortenwesen, dem Amtsblatt des Bundessortenamtes, veröffentlicht. Die saatgutrechtliche Sortenzulassung ist Voraussetzung für die Anerkennung von Re- benpflanzgut und diese wiederum ist im Sinne des Verbraucherschutzes Voraussetzung für das Inverkehrbringen. Pflanzgut einer in einem Vertragsstaat zugelassenen Rebsorte samt deren eingetragenen Klonen kann auch in den anderen Vertragsstaaten anerkannt und als anerkanntes Pflanzgut in Verkehr gebracht werden. -

Vine Culture in Emerging Wine Regions of Northern and Eastern Europe - Development, Varieties and Possibilities
Vine Culture in emerging wine regions of Northern and Eastern Europe - development, varieties and possibilities- Anja Antes ANTES Weinbau Service GmbH - grafting and winegrowing Agenda • Introduction • Development of Viticulture in Eastern and Northern Europe • Varieties • established • new possibilities • Worth mentioning • Summary Antes nurseries and winegrowing Antes nurseries and winegrowing • 1952: foundation by Vinzenz Antes • 1994: handover to Reinhard and Helmut Antes • 2014: enter of Anja Antes Antes nurseries and winegrowing • 43 ha (106 acre) vineyards / reproduction fields • comparisons of over 400 varieties and clones • table grapes • Rootstocks • 1.400.000 sold plants / year • working together with German viticulture institutes: Geisenheim, Freiburg, Weinsberg Export share 2013 Germany abroad Export Countries + Canada projects + China + Vietnam + Thailand Export to more than 30 countries (focus on emerging wine regions of Northern and Eastern Europe) Antes nurseries and winegrowing • Complete Service • supplies for vineyards (poles, wires, tubes…) • consulting and planning (soil-analysis, …) • project management und realisation Winnica Srebrna Góra (Winery Silberberg, Krakow) Vineyard at Silver Mountain Arildsvingaard, Sweden Biggest winery in Sweden Extreme rising number of plantings WHY rising number of plantings? - Climate Change - Chances of laws: legal possibility to grow and to sell wine - Interest in (new) culture – hope to earn money with this (new) type of agriculture - State subsidy - trend to drink regional wines