Caryopsis Micromorphological Survey of the Genus Themeda (Poaceae) and Allied Spathaceous Genera in the Andropogoneae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Poaceae Phytoliths from the Niassa Rift, Mozambique
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/222149229 Poaceae phytoliths from the Niassa Rift, Mozambique Article in Journal of Archaeological Science · August 2010 DOI: 10.1016/j.jas.2010.03.001 CITATIONS READS 44 409 9 authors, including: Fernando Astudillo Mary Barkworth Universidad San Francisco de Quito (USFQ) Utah State University 4 PUBLICATIONS 45 CITATIONS 81 PUBLICATIONS 902 CITATIONS SEE PROFILE SEE PROFILE Tim Aaron Bennett Chris Esselmont 8 PUBLICATIONS 242 CITATIONS The University of Calgary 6 PUBLICATIONS 161 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Stipeae (no longer a major focus) View project Grasses in North America View project All content following this page was uploaded by Rahab N Kinyanjui on 19 March 2018. The user has requested enhancement of the downloaded file. Journal of Archaeological Science 37 (2010) 1953e1967 Contents lists available at ScienceDirect Journal of Archaeological Science journal homepage: http://www.elsevier.com/locate/jas Poaceae phytoliths from the Niassa Rift, Mozambique Julio Mercader a,*, Fernando Astudillo a, Mary Barkworth b, Tim Bennett a, Chris Esselmont c, Rahab Kinyanjui d, Dyan Laskin Grossman a, Steven Simpson a, Dale Walde a a Department of Archaeology, University of Calgary, 2500 University Drive N.W., Calgary, Alberta T2N 1N4, Canada b Intermountain Herbarium, Utah State University, 5305 Old Main Hill, Logan, UH 84322-5305, USA c Environics Research Group, 999 8th Street S.W., Calgary, Alberta T2R 1J5, Canada d National Museum of Kenya, Department of Earth Sciences, Palynology and Paleobotany Section, P.O. -

Phytosociological Surveys and Monitoring of the Bromatological
European Journal of Nutrition & Food Safety 13(3): 54-61, 2021; Article no.EJNFS.68728 ISSN: 2347-5641 Phytosociological Surveys and Monitoring of the Bromatological Parameters According to the Age of Regrowth of Savannah Pastures Perfectly Reconstituted in the Central Zone of Côte d'Ivoire Gouagoua Séverin Kouadja1, Adam Camille Kouamé1*, Kouakou Eugène Kouadio1, Brou Jean Kouao1 and N’Gouan Cyrille Kouassi1 1Program of Animal Production, National Centre for Agricultural Research (CNRA), Bouaké Regional Office, P.O. box 633 Bouaké 01, Bouaké, Côte d’Ivoire. Authors’ contributions This work was carried out in collaboration among all authors. Author GSK is the principal investigator of this research. Author GSK was responsible for the collection of samples and contributed to the chemical analyses. Author Adam Camille KOUAMÉ contributed to the analyses and writing of the Manuscript. Author NCK participated in the design of the study. Author BJK was responsible for the formulation of the research question and reviewed the manuscript. Author KE reviewed the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJNFS/2021/v13i330390 Editor(s): (1) Dr. Kristina Mastanjevic, University of Osijek, Croatia. Reviewers: (1) Alsaied Alnaimy Habeeb, Nuclear Research Center, Egypt. (2) basem soudy, Cairo University, Egypt. Complete Peer review History: http://www.sdiarticle4.com/review-history/68728 Received 25 March 2021 Original Research Article Accepted 31 May 2021 Published 08 June 2021 ABSTRACT Although pastures in central (Affouvansou) Côte d'Ivoire are abundant, signs of undernutrition are observed in the animals towards the end of wintering, possibly due to insufficient quality pastures, poor grass quality, or poor herd distribution. -

Grass Genera in Townsville
Grass Genera in Townsville Nanette B. Hooker Photographs by Chris Gardiner SCHOOL OF MARINE and TROPICAL BIOLOGY JAMES COOK UNIVERSITY TOWNSVILLE QUEENSLAND James Cook University 2012 GRASSES OF THE TOWNSVILLE AREA Welcome to the grasses of the Townsville area. The genera covered in this treatment are those found in the lowland areas around Townsville as far north as Bluewater, south to Alligator Creek and west to the base of Hervey’s Range. Most of these genera will also be found in neighbouring areas although some genera not included may occur in specific habitats. The aim of this book is to provide a description of the grass genera as well as a list of species. The grasses belong to a very widespread and large family called the Poaceae. The original family name Gramineae is used in some publications, in Australia the preferred family name is Poaceae. It is one of the largest flowering plant families of the world, comprising more than 700 genera, and more than 10,000 species. In Australia there are over 1300 species including non-native grasses. In the Townsville area there are more than 220 grass species. The grasses have highly modified flowers arranged in a variety of ways. Because they are highly modified and specialized, there are also many new terms used to describe the various features. Hence there is a lot of terminology that chiefly applies to grasses, but some terms are used also in the sedge family. The basic unit of the grass inflorescence (The flowering part) is the spikelet. The spikelet consists of 1-2 basal glumes (bracts at the base) that subtend 1-many florets or flowers. -
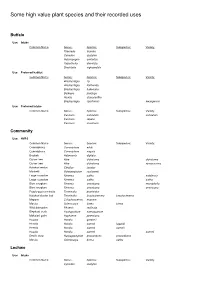
Some High Value Plant Species and Their Recorded Uses
Some high value plant species and their recorded uses Buffalo Use: Intake Common Name Genus: Species: Subspecies: Variety: Themeda triandra Cynodon dactylon Heteropogon contortus Hyperthelia dissoluta Brachiaria nigropedata Use: Preferred habitat Common Name Genus: Species: Subspecies: Variety: Brachystegia sp. Brachystegia floribunda Brachystegia bakeriana Baikiaea plurijuga Acacia ataxacantha Brachystegia spiciformis kwangensis Use: Preferred intake Common Name Genus: Species: Subspecies: Variety: Panicum coloratum coloratum Panicum repens Panicum maximum Community Use: HVPS Common Name Genus: Species: Subspecies: Variety: Commiphora Commiphora wildii Commiphora Commiphora virgata Baobab Adansonia digitata Quiver tree Aloe dichotoma dichotoma Quiver tree Aloe dichotoma ramosissima Kalahari melon Citrullus lanatus Manketti Schinziophyton rautanenii Large sourplum Ximenia caffra natalensis Large sourplum Ximenia caffra caffra Blue sourplum Ximenia americana microphylla Blue sourplum Ximenia americana americana Purple-pod terminalia Terminalia prunioides Kalahari cluster leaf Terminalia brachystemma brachystemma Mopane Colophospermu mopane Marula Sclerocarya birrea birrea Wild date palm Phoenix reclinata Elephant trunk Pachypodium namaquanum Makalani palm Hyphaene petersiana Hoodia Hoodia gordonii Hoodia Hoodia currorii lugardii Hoodia Hoodia currorii currorii Hoodia Hoodia currorii currorii Devil's claw Harpagophytum procumbens procumbens Marula Sclerocarya birrea caffra Lechwe Use: Intake Common Name Genus: Species: Subspecies: Variety: -

(Poaceae: Andropogoneae): a Presumed Extinct Grass from Andhra Pradesh, India
Phytotaxa 497 (2): 147–156 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2021 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.497.2.7 Rediscovery of Parahyparrhenia bellariensis (Poaceae: Andropogoneae): A presumed extinct grass from Andhra Pradesh, India SHAHID NAWAZ LANDGE1* & RAJENDRA D. SHINDE2 1 The Blatter Herbarium (BLAT), St. Xavier’s College (Autonomous), Mumbai 400001. �[email protected]; https://orcid.org/0000-0002-6734-6749 2 St. Xavier’s College (Autonomous), Mumbai 400001. �[email protected]; https://orcid.org/0000-0002-9315-9883 *Corresponding author: �[email protected] Abstract Parahyparrhenia bellariensis, an extremely rare and highly narrow endemic grass, has been rediscovered after almost 184 years from Cuddapah [Kadapa] district, Andhra Pradesh. The first description of its complete habit, basal portion and other features of the spikelets are provided along with new locality of its occurrence. In addition, photographs of the habitats, live plants, and a key to distinguish two Indian endemic species, distribution map and illustration are provided. As per the IUCN Red List Criteria this species is assessed here as Critically Endangered (CR). In order to facilitate the prospective conservation of this grass, we have discussed about the peculiarity of its habitat. Keywords: Eastern Ghats, Endemic, Gandikota Fort Hill, Gooty fort hill, Peninsular India, Robert Wight Introduction Parahyparrhenia A. Camus (1950: 404) is a fairly small, Afro-Eurasian genus, belonging to the tribe Andropogoneae Dumortier (1824: 84). It comprises seven species distributed in Africa and Tropical Asia, of which two species, namely, P. bellariensis (Hackel 1885: 123) Clayton (1972: 448) and recently described P. -

Policies and Incentives for Managing the Miombo Woodlands of Southern Africa
MANAGING THE MIOMBO WOODLANDS OF SOUTHERN AFRICA POLICIES, INCENTIVES, AND OPTIONS FOR THE RURAL POOR Disclaimer This work was originally prepared for the World Bank Africa Region’s Environment and Natural Resources Management Unit in 2008 to inform its regional work on forests. Extracts were subsequently published in the Journal of Natural Resources Policy Research (Vol.2, No. 1, 57-73) in January 2010. This volume was subsequently peer reviewed, revised, and has been modestly updated. The technical annexes, available online at http://www. profor.info/profor/content/miombo-annexes, have also been revised and updated, on the basis of peer review. All omissions and inaccuracies in this document are the responsibility of the authors. The views expressed do not necessarily represent those of the institutions involved, nor do they necessarily represent offi cial policies of PROFOR or the World Bank. Suggested citation: Dewees, P., B. Campbell, Y. Katerere, A. Sitoe, A.B. Cunningham, A. Angelsen and S. Wunder. 2011. Managing the Miombo Woodlands of Southern Africa: Policies, incentives, and options for the rural poor. Washington DC: Program on Forests (PROFOR). Published in November 2011 Printed on 100% Recycled paper Material in this book can be copied and quoted freely provided acknowledgement is given For a full list of publications please contact: Program on Forests (PROFOR) 1818 H Street, NW Washington, DC 20433, USA [email protected] www.profor.info/knowledge NOVEMBER 2011 MANAGING THE MIOMBO WOODLANDS OF SOUTHERN AFRICA POLICIES, INCENTIVES, AND OPTIONS FOR THE RURAL POOR Authors: Peter A. Dewees Bruce M. Campbell Yemi Katerere Almeida Sitoe Anthony B. -

Vegetation Survey of Batavia Downs, Cape York Peninsula
QR91003 Vegetation survey of Batavia Downs Cape York Peninsula V. J. Neldner, J. R. Clarkson Botany Branch Department of Primary Industries & Brisbane Queensland Government Technical Report This report is a scanned copy and some detail may be illegible or lost. Before acting on any information, readers are strongly advised to ensure that numerals, percentages and details are correct. This report is intended to provide information only on the subject under review. There are limitations inherent in land resource studies, such as accuracy in relation to map scale and assumptions regarding socio-economic factors for land evaluation. Before acting on the information conveyed in this report, readers should ensure that they have received adequate professional information and advice specific to their enquiry. While all care has been taken in the preparation of this report neither the Queensland Government nor its officers or staff accepts any responsibility for any loss or damage that may result from any inaccuracy or omission in the information contained herein. © State of Queensland 1991 For information about this report contact [email protected] Research Establishments Publication QR91003 Vegetation survey of Batavia Downs Cape York Peninsula V. J. Neldner, J. R. Clarkson Botany Branch Department of Primary Industries Brisbane ISSN 0813-4391 Agdex 301/06 This publication was prepared for officers of the Department of Primary Industries. It may be distributed to other interested individuals and organisations. © Queensland Government 1991 Department of Primary Industries, Queensland GPO Box 46 Brisbane Qld4001 Ill Contents List of figures Page iv List of tables iv List of plates iv Summary v 1. -

Using the Checklist N W C
Using the checklist • The arrangement of the checklist is alphabetical by family followed by genus, grouped under Pteridophyta, Gymnosperms, Monocotyledons and Dicotyledons. • All species and synonyms are arranged alphabetically under genus. • Accepted names are in bold print while synonyms or previously-used names are in italics. • In the case of synonyms, the currently used name follows the equals sign (=), and only refers to usage in Zimbabwe. • Distribution information is included under the current name. • The letters N, W, C, E, and S, following each listed taxon, indicate the known distribution of species within Zimbabwe as reflected by specimens in SRGH or cited in the literature. Where the distribution is unknown, we have inserted Distr.? after the taxon name. • All species known or suspected to be fully naturalised in Zimbabwe are included in the list. They are preceded by an asterisk (*). Species only known from planted or garden specimens were not included. Mozambique Zambia Kariba Mt. Darwin Lake Kariba N Victoria Falls Harare C Nyanga Mts. W Mutare Gweru E Bulawayo GREAT DYKEMasvingo Plumtree S Chimanimani Mts. Botswana N Beit Bridge South Africa The floristic regions of Zimbabwe: Central, East, North, South, West. A checklist of Zimbabwean vascular plants A checklist of Zimbabwean vascular plants edited by Anthony Mapaura & Jonathan Timberlake Southern African Botanical Diversity Network Report No. 33 • 2004 • Recommended citation format MAPAURA, A. & TIMBERLAKE, J. (eds). 2004. A checklist of Zimbabwean vascular plants. -

Grasslands of the World.Pdf
The views expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Food and Agriculture Organization of the United Nations. The mention or omission of specific companies, their products or brand names does not imply any endorsement or judgement by the Food and Agriculture Organization of the United Nations. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. ISBN 92-5-105337-5 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] © FAO 2005 CONTENTS Foreword xiii Acknowledgements xv Contributors xvii Glossary of technical terms and abbreviations used in the text xviii Chapter 1 – Introduction 1 Purpose of the book 13 Structure of the book 13 Complementary information resources 16 References 17 Chapter 2 – The changing face of pastoral systems in grass-dominated ecosystems of eastern Africa 19 R.S. -

Phylogenetics of Andropogoneae (Poaceae: Panicoideae) Based on Nuclear Ribosomal Internal Transcribed Spacer and Chloroplast Trnl–F Sequences Elizabeth M
Aliso: A Journal of Systematic and Evolutionary Botany Volume 23 | Issue 1 Article 40 2007 Phylogenetics of Andropogoneae (Poaceae: Panicoideae) Based on Nuclear Ribosomal Internal Transcribed Spacer and Chloroplast trnL–F Sequences Elizabeth M. Skendzic University of Wisconsin–Parkside, Kenosha J. Travis Columbus Rancho Santa Ana Botanic Garden, Claremont, California Rosa Cerros-Tlatilpa Rancho Santa Ana Botanic Garden, Claremont, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Skendzic, Elizabeth M.; Columbus, J. Travis; and Cerros-Tlatilpa, Rosa (2007) "Phylogenetics of Andropogoneae (Poaceae: Panicoideae) Based on Nuclear Ribosomal Internal Transcribed Spacer and Chloroplast trnL–F Sequences," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 23: Iss. 1, Article 40. Available at: http://scholarship.claremont.edu/aliso/vol23/iss1/40 Aliso 23, pp. 530–544 ᭧ 2007, Rancho Santa Ana Botanic Garden PHYLOGENETICS OF ANDROPOGONEAE (POACEAE: PANICOIDEAE) BASED ON NUCLEAR RIBOSOMAL INTERNAL TRANSCRIBED SPACER AND CHLOROPLAST trnL–F SEQUENCES ELIZABETH M. SKENDZIC,1,3,4 J. TRAVIS COLUMBUS,2 AND ROSA CERROS-TLATILPA2 1University of Wisconsin–Parkside, 900 Wood Road, Kenosha, Wisconsin 53141-2000, USA; 2Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711-3157, USA 3Corresponding author ([email protected]) ABSTRACT Phylogenetic relationships among 85 species representing 35 genera in the grass tribe Andropogo- neae were estimated from maximum parsimony and Bayesian analyses of nuclear ITS and chloroplast trnL–F DNA sequences. Ten of the 11 subtribes recognized by Clayton and Renvoize (1986) were sampled. Independent analyses of ITS and trnL–F yielded mostly congruent, though not well resolved, topologies. -

Fire: Its Effect on Grasslands, Including Swamps-Southern, Central and Eastern Africa
Fire: Its Effect on Grasslands, Including Swamps-Southern, Central and Eastern Africa H. J. VAN RENSBURG Range Ecologist, F. A. O. Gabrone, Botsrwana* INTRODUCTION THE aspects of fire described in this paper relate to a very large area. The contents are based on the results from experi ments and observations carried out in Southern, Central and Eastern Africa for many years. Conditions vary greatly, climatically, topographically and in vege tation types and soils. They range from coastal regions with variable grassland-woodland formations to high altitude mountain grassland and woodland associations. There are extensive inland plateaux sup porting mixed vegetation communities. There are extensive flood plains associated with large drainage systems. These are subjected to various degrees of inundation. Some flood plain areas are rela tively dry while others are under permanent swamp and lagoon. Due to the extent and complexity of this subject, it is only pos sible to deal with relatively few aspects . .. Current address: Pasture & Fodder Crops Branch, Plant Production & Protec tion Division, F.A.O., Rome, Italy. 175 H. J. VAN RENSBURG Fires are cleansers as well as destroyers. Nature gives of her bounty and if man does not use it to the utmost, he is left with a residue that, in course of time, becomes a hazard. In most parts of Africa there is no rain for about 8 months of the year. People wait and pray for rain so that crops and pastures may grow. Growth is rapid during the rainy season. Herbage, surplus to grazing requirements, should be converted into hay and silage while it is still young and nutritious. -

Classification and Biogeography of Panicoideae (Poaceae) in the New World Fernando O
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Scholarship@Claremont Aliso: A Journal of Systematic and Evolutionary Botany Volume 23 | Issue 1 Article 39 2007 Classification and Biogeography of Panicoideae (Poaceae) in the New World Fernando O. Zuloaga Instituto de Botánica Darwinion, San Isidro, Argentina Osvaldo Morrone Instituto de Botánica Darwinion, San Isidro, Argentina Gerrit Davidse Missouri Botanical Garden, St. Louis Susan J. Pennington National Museum of Natural History, Smithsonian Institution, Washington, D.C. Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Zuloaga, Fernando O.; Morrone, Osvaldo; Davidse, Gerrit; and Pennington, Susan J. (2007) "Classification and Biogeography of Panicoideae (Poaceae) in the New World," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 23: Iss. 1, Article 39. Available at: http://scholarship.claremont.edu/aliso/vol23/iss1/39 Aliso 23, pp. 503–529 ᭧ 2007, Rancho Santa Ana Botanic Garden CLASSIFICATION AND BIOGEOGRAPHY OF PANICOIDEAE (POACEAE) IN THE NEW WORLD FERNANDO O. ZULOAGA,1,5 OSVALDO MORRONE,1,2 GERRIT DAVIDSE,3 AND SUSAN J. PENNINGTON4 1Instituto de Bota´nica Darwinion, Casilla de Correo 22, Labarde´n 200, San Isidro, B1642HYD, Argentina; 2([email protected]); 3Missouri Botanical Garden, PO Box 299, St. Louis, Missouri 63166, USA ([email protected]); 4Department of Botany, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20013-7012, USA ([email protected]) 5Corresponding author ([email protected]) ABSTRACT Panicoideae (Poaceae) in the New World comprise 107 genera (86 native) and 1357 species (1248 native).