Melanoma Arising in Association with Blue Nevus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
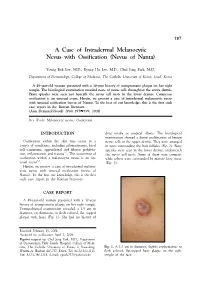
A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta)
197 A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta) Young Bok Lee, M.D., Kyung Ho Lee, M.D., Chul Jong Park, M.D. Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea A 49-year-old woman presented with a 30-year history of asymptomatic plaque on her right temple. The histological examination revealed nests of nevus cells throughout the entire dermis. Bony spicules were seen just beneath the nevus cell nests in the lower dermis. Cutaneous ossification is an unusual event. Herein, we present a case of intradermal melanocytic nevus with unusual ossification (nevus of Nanta). To the best of our knowledge, this is the first such case report in the Korean literature. (Ann Dermatol (Seoul) 20(4) 197∼199, 2008) Key Words: Melanocytic nevus, Ossification INTRODUCTION drug intake or medical illness. The histological examination showed a dense proliferation of benign Ossification within the skin may occur in a nevus cells in the upper dermis. They were arranged variety of conditions, including pilomatricoma, basal in nests surrounding the hair follicles (Fig. 2). Bony cell carcinoma, appendageal and fibrous prolifera- spicules were seen in the lower dermis, underneath 1,2 tion, inflammation and trauma . The occurrence of the nevus cell nests. Some of them were compact ossification within a melanocytic nevus is an un- while others were surrounded by mature fatty tissue 3-5 usual event . (Fig. 3). Herein, we present a case of intradermal melano- cytic nevus with unusual ossification (nevus of Nanta). To the best our knowledge, this is the first such case report in the Korean literature. -

Two Cases of Nevoid Basal Cell Carcinoma Syndrome in One Family
221 Two Cases of Nevoid Basal Cell Carcinoma Syndrome in One Family Dong Jin Ryu, M.D., Yeon Sook Kwon, M.D., Mi Ryung Roh, M.D., Min-Geol Lee, M.D., Ph.D. Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea The nevoid basal cell carcinoma syndrome, or Gorlin-Goltz syndrome, is an autosomal dominant multiple system disorder with high penetrance and variable expressions, although it can also arise spontaneously. The diagnostic criteria for nevoid basal cell carcinoma syndrome include multiple basal cell carcinomas, palmoplantar pits, multiple odontogenic keratocysts, skeletal anomalies, positive family history, ectopic calcification and neurological anomalies. We report a brother and sister who were both diagnosed with nevoid basal cell carcinoma syndrome. (Ann Dermatol (Seoul) 20(4) 221∼225, 2008) Key Words: Basal cell carcinoma, Nevoid basal cell carcinoma syndrome, Odontogenic keratocyst INTRODUCTION cell carcinoma syndrome. The nevoid basal cell carcinoma syndrome (NBCCS), or Gorlin-Goltz syndrome, is an auto- CASE REPORT somal dominant multiple system disorder with high 1 penetrance and variable expressions . However, Case 1 60% of patients with NBCCS are sporadic cases. It An 11-year-old male was referred to our depart- has an estimated prevalence of 1 in 60,000 with ment for the evaluation of multiple miliary sized 2 equal distributions among males and females . The pigmented macules on the palm and sole that had well-defined diagnostic criteria include cutaneous increased in number over several years. He had an anomalies, dento-facial anomalies, skeletal ano- operation for inguinal hernia at 3 years of age, but malies, positive family history, neurological ano- no other medical problems. -

Acquired Bilateral Nevus of Ota–Like Macules (Hori's Nevus): a Case
Acquired Bilateral Nevus of Ota–like Macules (Hori’s Nevus): A Case Report and Treatment Update Jamie Hale, DO,* David Dorton, DO,** Kaisa van der Kooi, MD*** *Dermatology Resident, 2nd year, Largo Medical Center/NSUCOM, Largo, FL **Dermatologist, Teaching Faculty, Largo Medical Center/NSUCOM, Largo, FL ***Dermatopathologist, Teaching Faculty, Largo Medical Center/NSUCOM, Largo, FL Abstract This is a case of a 71-year-old African American female who presented with bilateral periorbital hyperpigmentation. After failing treatment with a topical retinoid and hydroquinone, a biopsy was performed and was consistent with acquired bilateral nevus of Ota-like macules, or Hori’s nevus. A review of histopathology, etiology, and treatment is discussed below. cream and tretinoin 0.05% gel. At this visit, a Introduction Figure 2 Acquired nevus of Ota-like macules (ABNOM), punch biopsy of her left zygoma was performed. or Hori’s nevus, clinically presents as bilateral, Histopathology reported sparse proliferation blue-gray to gray-brown macules of the zygomatic of irregularly shaped, haphazardly arranged melanocytes extending from the superficial area. It less often presents on the forehead, upper reticular dermis to mid-deep reticular dermis outer eyelids, and nose.1 It is most common in women of Asian descent and has been reported Figure 4 in ages 20 to 70. Classically, the eye and oral mucosa are uninvolved. This condition is commonly misdiagnosed as melasma.1 The etiology of this condition is not fully understood, and therefore no standardized treatment has been Figure 3 established. Case Report A 71-year-old African American female initially presented with a two week history of a pruritic, flaky rash with discoloration of her face. -

Short Course 11 Pigmented Lesions of the Skin
Rev Esp Patol 1999; Vol. 32, N~ 3: 447-453 © Prous Science, SA. © Sociedad Espafiola de Anatomfa Patol6gica Short Course 11 © Sociedad Espafiola de Citologia Pigmented lesions of the skin Chairperson F Contreras Spain Ca-chairpersons S McNutt USA and P McKee, USA. Problematic melanocytic nevi melanin pigment is often evident. Frequently, however, the lesion is solely intradermal when it may be confused with a fibrohistiocytic RH. McKee and F.R.C. Path tumor, particularly epithelloid cell fibrous histiocytoma (4). It is typi- cally composed of epitheliold nevus cells with abundant eosinophilic Brigham and Women’s Hospital, Harvard Medical School, Boston, cytoplasm and large, round, to oval vesicular nuclei containing pro- USA. minent eosinophilic nucleoli. Intranuclear cytoplasmic pseudoinclu- sions are common and mitotic figures are occasionally present. The nevus cells which are embedded in a dense, sclerotic connective tis- Whether the diagnosis of any particular nevus is problematic or not sue stroma, usually show maturation with depth. Less frequently the nevus is composed solely of spindle cells which may result in confu- depends upon a variety of factors, including the experience and enthusiasm of the pathologist, the nature of the specimen (shave vs. sion with atrophic fibrous histiocytoma. Desmoplastic nevus can be distinguished from epithelloid fibrous histiocytoma by its paucicellu- punch vs. excisional), the quality of the sections (and their staining), larity, absence of even a focal storiform growth pattern and SiQO pro- the hour of the day or day of the week in addition to the problems relating to the ever-increasing range of histological variants that we tein/HMB 45 expression. -

Co-Occurrence of Vitiligo and Becker's Nevus: a Case Report
Case Report Olgu Sunumu DOI: 10.4274/turkderm.71354 Turkderm - Arch Turk Dermatol Venerology 2016;50 Co-occurrence of vitiligo and Becker's nevus: A case report Vitiligo ve Becker nevüs birlikteliği: Olgu sunumu Ayşegül Yalçınkaya İyidal, Özge Çokbankir*, Arzu Kılıç** Ağrı State Hospital, Clinic of Dermatology, *Clinic of Pathology, Ağrı, Turkey **Balıkesir University Faculty of Medicine, Department of Dermatology, Balıkesir, Turkey Abstract Vitiligo is an acquired disorder with an unknown etiology in which genetic and non-genetic factors coexist. Melanocytes are destructed in the affected skin areas and clinically depigmented macules and patches appear on the skin. Becker's nevus (BN) appears as hyperpigmented macule, patch or verrucous plaques with sharp and irregular margins and often unilateral occurrence and with associated hypertrichosis in various degrees. Although its pathogenesis is unknown, it is suggested to represent a hamartomatous lesion harboring androgen receptors on the lesion. In this report, we present a 19-year-old male patient who developed vitiligo lesions and then BN adjacent to the vitiligo lesion in the right upper back portion of the body ten years after the initial vitiligo lesion. Keywords: Becker's nevus, vitiligo, co-occurrence Öz Vitiligo nedeni tam olarak bilinmeyen, genetik ve genetik olmayan faktörlerin birlikte rol oynadığı edinsel bir bozukluktur. Bu hastalıkta tutulan deride melanositler ortadan kalkar, klinik olarak depigmente makül ve yamalar belirir. Becker nevüs (BN) sıklıkla unilateral dağılım gösteren, keskin ama düzensiz sınırlı hiperpigmente makül, yama veya verrüköz plakların izlendiği, üzerinde değişik derecelerde hipertrikozun bulunduğu bir hastalıktır. Patogenezi belli olmamakla birlikte hamartamatöz bir lezyon olduğu ve üzerinde androjen reseptörlerinin arttığı ileri sürülmektedir. -

Oral Pathology
Oral Pathology Palatal blue nevus in a child Catherine M. Flaitz DDS, MS Georgeanne McCandless DDS Dr. Flaitz is professor, Oral and Maxillofacial Pathology and Pediatric Dentistry, Department of Stomatology, University of Texas at Houston Health Science Center Dental Branch; Dr. McCandless has a private practice in The Woodlands, TX. Correspond with Dr. Flaitz at [email protected] Abstract The intraoral blue nevus occurs infrequently in children. This by the labial mucosa (1). Intraoral lesions have a predilection case report describes the clinical features of an acquired blue ne- for females in the third and fourth decades, in contrast to cu- vus in a 7 year-old girl that involved the palatal mucosa. A taneous lesions that normally develop in children. In large differential diagnosis and justification for surgical excision of this biopsy series, only 2% of the oral blue nevi are diagnosed in oral lesion are discussed. (Pediatr Dent 23:354-355, 2001) children and adolescents (1). Similar to their cutaneous coun- terpart, most oral lesions are acquired; however, there are ith the exception of vascular entities, neoplastic isolated reports of congenital examples. lesions with a blue discoloration are an unusual find Clinically, most lesions present as a solitary blue, gray or Wing in children. Although the blue nevus is a blue-black macule or slightly raised nodule that measures less relatively common finding of the skin in the pediatric popula- than 6 mm in size. The margins are often regular but indis- tion, only a few intraoral examples are documented in the tinct and the surface is smooth. -

Dermoscopy on Nevus Comedonicus: a Case Report and Review of the Literature
Case report Dermoscopy on nevus comedonicus: a case report and review of the literature Grażyna Kamińska-Winciorek 1, Radosław Śpiewak 2 1The Center for Cancer Prevention and Treatment, Katowice, Poland Head: Beata Wydmańska 2Department of Experimental Dermatology and Cosmetology, Faculty of Pharmacy, Jagiellonian University Medical College, Krakow, Poland Head: Prof. Radosław Śpiewak MD. PhD Postep Derm Alergol 2013; XXX, 4: 252 –254 DOI: 10.5114/pdia.2013.37036 Abstract Nevus comedonicus (NC) is a very rare, benign hamartoma characterised by the occurrence of dilated, comedo-like openings, typically on the face, neck, upper arms, chest or abdomen. In uncertain cases, histopathological exami - nation confirms the diagnosis. The authors suggest dermoscopy as a rapid and useful method of initial diagnosis of nevus comedonicus based upon its distinctive dermoscopic features. The dermoscopy reveals numerous light- and dark-brown, circular or barrel-shaped, homogenous areas with prominent keratin plugs. Key words: dermoscopy, dermatoscopy, nevus comedonicus, epidermal nevus, acne vulgaris. Introduction a hypopigmented, slightly hypotrophic, linear spot of Nevus comedonicus (NC) is a benign hamartoma cha - 2 cm × 8 cm (Figure 1). The plugs could not be extracted racterised by the occurrence of dilated comedo-like open - mechanically. The dermoscopic examination revealed ings, with black or brown keratin plugs, typically localised the distinctive pattern consisting of dark, sharply demar - on the face, neck, upper arms, chest or abdomen. The diag - cated keratin plugs of 1–3 mm diameter, numerous struc - nosis of nevus comedonicus is relatively easy. In uncertain tureless, circular- and barrel-shaped, homogenous areas cases, a typical histopathological picture confirms the diag - with hyperkeratotic plugs of various shades of brown nosis. -

Identification of HRAS Mutations and Absence of GNAQ Or GNA11
Modern Pathology (2013) 26, 1320–1328 1320 & 2013 USCAP, Inc All rights reserved 0893-3952/13 $32.00 Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi Ryan P Bender1, Matthew J McGinniss2, Paula Esmay1, Elsa F Velazquez3,4 and Julie DR Reimann3,4 1Caris Life Sciences, Phoenix, AZ, USA; 2Genoptix Medical Laboratory, Carlsbad, CA, USA; 3Dermatopathology Division, Miraca Life Sciences Research Institute, Newton, MA, USA and 4Department of Dermatology, Tufts Medical Center, Boston, MA, USA HRAS is mutated in B15% of Spitz nevi, and GNAQ or GNA11 is mutated in blue nevi (46–83% and B7% respectively). Epithelioid blue nevi and deep penetrating nevi show features of both blue nevi (intradermal location, pigmentation) and Spitz nevi (epithelioid morphology). Epithelioid blue nevi and deep penetrating nevi can also show overlapping features with melanoma, posing a diagnostic challenge. Although epithelioid blue nevi are considered blue nevic variants, no GNAQ or GNA11 mutations have been reported. Classification of deep penetrating nevi as blue nevic variants has also been proposed, however, no GNAQ or GNA11 mutations have been reported and none have been tested for HRAS mutations. To better characterize these tumors, we performed mutational analysis for GNAQ, GNA11, and HRAS, with blue nevi and Spitz nevi as controls. Within deep penetrating nevi, none demonstrated GNAQ or GNA11 mutations (0/38). However, 6% revealed HRAS mutation (2/32). Twenty percent of epithelioid blue nevi contained a GNAQ mutation (2/10), while none displayed GNA11 or HRAS mutation. Eighty-seven percent of blue nevi contained a GNAQ mutation (26/30), 4% a GNA11 mutation (1/28), and none an HRAS mutation. -

The Role of Androgen Receptors in the Clinical Course of Nevus Sebaceus of Jadassohn Katherine S
The Role of Androgen Receptors in the Clinical Course of Nevus Sebaceus of Jadassohn Katherine S. Hamilton, M.D., Sandra Johnson, M.D., Bruce R. Smoller, M.D. Department of Pathology, Vanderbilt University Medical Center, Nashville, Tennessee (KSH); and Departments of Dermatology (SJ, BRS) and Pathology (SJ), University of Arkansas for Medical Services, Little Rock, Arkansas During puberty, they usually enlarge and become Nevus sebaceus of Jadassohn (NSJ) is a benign, con- elevated, verrucous, or nodular and may appear genital hamartoma that often presents at birth, ap- brown (1, 2). In late childhood and adulthood, there pears to regress in childhood, and grows during is a significant risk of developing a secondary tu- puberty, suggesting possible hormonal control. We mor, the most common of which are syringocysta- studied 18 cases of NSJ from children and adults for denoma papilliferum and basal cell carcinoma (1, immunohistochemical evidence of androgen recep- 3). Myriad other cutaneous appendageal neoplasms tor expression. The lesions were evaluated for loca- have also been reported to arise within NSJ. tion and pattern of immunostaining, and these Androgen receptors (AR) are nuclear ligand–de- findings were compared between age groups, sexes, pendent transcription factors of the steroid super- and to androgen receptor expression in normal family that bind testosterone and dihydroxytestos- skin. Androgen receptor positivity was seen in the terone (4). AR have been identified in normal sebaceous glands, in eccrine glands with and with- cutaneous structures and in some epithelial tu- out apocrine change, and rarely in keratinocytes in mors. In normal skin, AR have been localized to the sebaceous nevi. -

Choroidal Nevus Transformation Into Melanoma: Analysis of 2514 Consecutive Cases
CLINICAL SCIENCES Choroidal Nevus Transformation Into Melanoma Analysis of 2514 Consecutive Cases Carol L. Shields, MD; Minoru Furuta, MD; Edwina L. Berman, BS; Jonathan D. Zahler, MD; Daniel M. Hoberman, BS; Diep H. Dinh, BS; Arman Mashayekhi, MD; Jerry A. Shields, MD Objective: To determine features that are predictive of halo absence (P=.009). A mnemonic device to recall risk growth of choroidal nevi into melanoma. factors of ocular melanoma is “To find small ocular mela- noma using helpful hints,” representing thickness, fluid, Methods: This was a retrospective medical record re- symptoms, orange pigment, margin, ultrasonographic hol- view of 2514 consecutive eyes; Kaplan-Meier estimates lowness, and halo absence. The median hazard ratio for and Cox regression analyses were used. those with 1 to 2 risk factors was 3; for 3 or 4 factors, 5; for 5 to 6 factors, 9; and for all 7 factors, 21. Results: The median tumor basal diameter was 5.0 mm and thickness was 1.5 mm. Nevus growth into mela- Conclusions: In an analysis of 2514 choroidal nevi, fac- noma occurred in 2%, 9%, and 13% of eyes at 1, 5, and tors predictive of growth into melanoma included greater 10 years, respectively. Factors predictive of growth into thickness, subretinal fluid, symptoms, orange pigment, melanoma by multivariable analysis included tumor thick- margin near disc, and 2 new features: ultrasonographic ness greater than 2 mm (PϽ.001), subretinal fluid hollowness and absence of halo. (P =.002), symptoms (P =.002), orange pigment (PϽ.001), tumor margin within 3 mm of the optic disc (P=.001), ultrasonographic hollowness (PϽ.001), and Arch Ophthalmol. -

Differentiating Malignant Melanoma from Other Lesions Using Dermoscopy
PRAXIS Differentiating malignant melanoma from other lesions using dermoscopy Ahmed Mourad Robert Gniadecki MD PhD DMSci ermoscopy (also called dermatoscopy, epilumi- nescent microscopy, or episcopy) is a noninvasive Figure 1. Image depicting proper dermoscopic technique: method of examining skin lesions using a hand- This is a polarized light dermoscope and does not require Dheld magnifying device (a dermoscope) equipped with a direct contact with the skin or application of oil. light source.1 Dermoscopy allows adequate visualization of the structures in the skin not only by magnifying them but also by eliminating the surface light reflection and scatter that obscures the deeper features.2,3 This arti- cle provides information on the dermoscopic features specific to malignant melanoma and other pigmented lesions that often resemble malignant melanoma via naked-eye examination (ie, benign melanocytic nevus [BMN], seborrheic keratosis, and dermatofibroma). Technique Before evaluating the lesion of interest using dermoscopy, the clinician should take an adequate history and evalu- ate the morphology and distribution of the lesion with the naked eye.1 Dermoscopy is then performed by apply- ing the dermoscope on the lesion of interest and looking through the lens to visualize the morphologic features of The presence of 2 or more of the above features sug- the skin lesion (Figure 1). Dermoscopy should never be gests a suspicious lesion that should be biopsied or that used alone, and the dermoscopic result should be corre- the patient should be referred for further assessment.4 lated with that of the naked-eye examination. The cost of a dermoscope ranges from a few hundred Conditions to a few thousand dollars. -

Lumps & Bumps: Approach to Common Dermatologic Neoplasms
Case-Based Approach to Common Dermatologic Neoplasms Patrick Retterbush, MD, FAAD Mohs Surgery & Dermatologic Oncology Associate Member of the American College of Mohs Surgery Private Practice: Lockman Dermatology January 27th 2018 Disclosure of Relevant Financial Relationships • I do not have any relevant financial relationships, commercial interests, and/or conflicts of interest regarding the content of this presentation. Goals/Objectives • Recognize common benign growths • Recognize common malignant growths • Useful clues & examination for evaluating melanocytic nevi and when to be concerned for melanoma/atypical moles • How to perform a basic skin biopsy and which method/type to choose • Basic treatment/when to refer Key Questions & Physical Examination Findings for a Growth History Physical Examination • How long has the lesion been • Describing a growth present? – flat or raised? • flat – macule (<1cm) or patch (>1cm) – years, months, weeks • raised – papule (<1cm) or plaque (>1cm) – nodule if deep (majority of lesion in • Has it changed? dermis/SQ) – Size – secondary descriptive features • scaly (hyperkeratosis, retention of strateum – Shape corneum) – Color • crusty (dried serum, blood, or pus on surface) • eroded or ulcerated (partial vs. full thickness – Symptoms – pain, bleeding, itch? epidermal loss) – Over what time frame? • color (skin colored, red, pigmented, pearly) • feel (hard or soft, mobile or fixed) • PMH: • size: i.e. 6 x 4mm – prior skin cancers • Look at the rest of the skin/region of skin • SCC/BCCs vs. melanoma