Predictors of Treatment Response to Liraglutide in Type 2 Diabetes in a Real‑World Setting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Itinerario Ciclopedonale Lungo Il Fiume Brenta Da Pontevigodarzere a Carmignano Di Brenta
Itinerario ciclopedonale lungo il fiume Brenta da Pontevigodarzere a Carmignano di Brenta PREMESSA L’itinerario lungo il corso del fiume Brenta è motivato dalla presenza di un’area di notevole interesse ambientale che merita una sua specifica valorizzazione anche a livello di turismo minore e diffuso. Inoltre, il progetto ha tra gli obiettivi anche quello di favorire la mobilità ciclabile sia a livello locale - comunale che provinciale, evidenziando le possibili connessioni extraprovinciali. Infatti, è possibile creare, in un’area che interessa quattro province quali quelle di Venezia, Padova, Vicenza e Trento, un sistema integrato di percorsi che colleghino i principali centri urbani (Caldonazzo, Bassano, Cittadella, Limena, Chioggia,) ed i capoluoghi (Trento, Vicenza, Padova e Venezia) inseriti in un’area caratterizzata da valenze ambientali e paesaggistiche di primordine, passando dalle bellezze delle montagne trentine a quelle del litorale veneto. In ambito locale si risponde alla necessità di creare dei percorsi alternativi alle strade con traffico automobilistico, per esigenze di spostamenti a breve raggio e ragioni di sicurezza e salubrità; a più ampia scala si mira alla valorizzazione delle risorse naturali e culturali diffuse, rivolgendosi alla domanda cicloturistica dei week-end , delle escursioni e delle gite fuori porta, ma anche degli itinerari a media e lunga percorrenza con delle esigenze precise, quali: • aspetti divulgativi e segnalazione dei percorsi e delle risorse naturalistiche e culturali diffuse, che particolarmente la bicicletta consente di apprezzare; • strutture di accoglienza generalmente extra-alberghiere (ostelli, agriturismi, campeggi, spazio attrezzato per caravans, ecc) e di ristorazione alla ricerca di tipicità e tradizioni eno-gastronomiche locali; • potenziamento dell’intermodalità di trasporto con treno, autobus e mezzi di navigazione che consentano il trasporto delle bici. -
Diapositiva 1
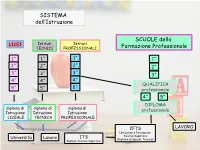
SISTEMA dell’Istruzione SCUOLE della LICEI Istituti Istituti TECNICI PROFESSIONALI Formazione Professionale 1^ 1^ 1^ 1^ 2^ 2^ 2^ 2^ 3^ 3^ 3^ 3^ 4^ 4^ 4^ QUALIFICA 5^ 5^ 5^ professionale 4^ 5^ DIPLOMA diploma di diploma di diploma di professionale Istruzione Istruzione Istruzione LICEALE TECNICA PROFESSIONALE IFTS LAVORO Istruzione e Formazione Tecnica Superiore Università Lavoro ITS (Diploma di special. Tecnica) Diploma Tecnico Superiore SCUOLA MEDIA LICEO PROFESSIONALE TECNICO SCUOLA della Formazione Professionale LICEO 5 ANNI MUSICALE SCIENTIFICO Tradizionale Scienze applicate Sportivo CLASSICO e CLASSICO EUROPEO Tradizionale SCIENZE UMANE A.u.r.e.u.s (competenze artistiche e linguistiche) Tradizionale Giuridico-economico Economico-Sociale Matematico-scientifico LINGUISTICO ARTISTICO Architettura e Ambiente Arti Figurative Scenografia DIPLOMA Design ISTRUZIONE Audiovisivo multimediale LICEALE Grafica Scienze Umane LICEI Duca d’Aosta - Padova Caro – Cittadella Cattaneo Mattei - Monselice Scientifico e Educandato San Benedetto – Montagnana Scientifico opz. Scienze Applicate Scienze Umane opz. Economico Sociale Alberti - Abano Duca d’Aosta - Padova Cornaro - Padova Marchesi - Padova Curiel - Padova Newton Pertini – Camposampiero Fermi – Padova Educandato San Benedetto – Montagnana Nievo - Padova Einstein – Piove di Sacco Galilei – Selvazzano Newton Pertini – Camposampiero Caro – Cittadella Ferrari – Este Cattaneo Mattei – Monselice Classico J. da Montagnana – Montagnana Tito Livio - Padova Rolando da Piazzola – Piazzola sul Brenta Caro -

Distretto Militare Di Padova- Ruoli Matricolari
Pe:.R K\C...M\Ei'6Efi!E. B,\JOl.0 ')\fì,8 O ... C\KE: 'b, \J\....'.) u fF1 �,R Lt-. o .::cl·-rn \ e P\\.E. 1 f<.IVoL�E.�..SI ?,F.,'f:.'5So L UFF1 -Gio- ----SICRI C ES(;..,:Zc., rio - \.J\� E1Rl)R 1� '1...:, o::d.8� Ro1if\ I lé.L. 06. 4-=f- ..55 �558 C'?. 4b 9-i 3-i 6 "::f ot0aR.1 F, ce� )tt:: \ \. J · �.,\ "Ft::.:.A CASE�MPt N�tAK O '......iA ' { · ,. U\f\ �?t'R\Ol?ì dLt RorlA o�. C..1- 3581.0b MINISTERO PER I BENI CULTURALI E AMBIENTALI ARCHIVIO DI STATO DI PADOVA UFFICIO LEVA DI PADOVA - DISTRETTO DI PADOVA Le liste di leva di Padova risultano divise per mandamento e per classe. Vi sono compresi anche i volumi relativi alle liste di revisioni dei riformati e rivedibili di Padova e provincia . 1 mandamenti (n.9) comprendono a loro volta un certo numero di comuni secondo la seguente suddivisione: 1. Mandamento di Camposampiero. Comprende i seguenti 15 comuni: · Borgoricco San Giorgio delle Pe1tiche Campo San Martino San Michele delle Badesse Campodarsego Sant'Eufemia Camposampiero Santa Giustina in Colle Cmtarolo Trebaseleghe · Loreggia Villa del Conte Massanzago Villanova di Camposampiero Piombino Dese - 2. Mandamento di Cittadella. Comprende i seguenti 10 comuni: Cittadella Gazzo Padovano Carmignano di Brenta San Giorgio in Bosco Fontaniva San Martino di Lupari Galliera Veneta San Pietro in Gù Grantorto Tombolo Classe 1857: Mandamento di CAMPOSAMPIERO n. 100 Mandamento di CITTADELLA n. 101 Mandamento di CONSELVE n. 102 Mandamento di ESTE n. 103 Mandamento di MONSELICE n. -
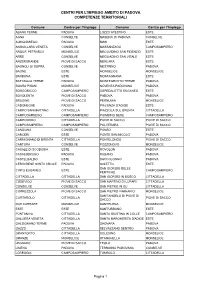
Centri Per L'impiego Ambito Di Padova Competenze Territoriali
CENTRI PER L'IMPIEGO AMBITO DI PADOVA COMPETENZE TERRITORIALI Comune Centro per l’Impiego Comune Centro per l’Impiego ABANO TERME PADOVA LOZZO ATESTINO ESTE AGNA CONSELVE MASERA' DI PADOVA CONSELVE ALBIGNASEGO PADOVA MASI ESTE ANGUILLARA VENETA CONSELVE MASSANZAGO CAMPOSAMPIERO ARQUA' PETRARCA MONSELICE MEGLIADINO SAN FIDENZIO ESTE ARRE CONSELVE MEGLIADINO SAN VITALE ESTE ARZERGRANDE PIOVE DI SACCO MERLARA ESTE BAGNOLI DI SOPRA CONSELVE MESTRINO PADOVA BAONE ESTE MONSELICE MONSELICE BARBONA ESTE MONTAGNANA ESTE BATTAGLIA TERME PADOVA MONTEGROTTO TERME PADOVA BOARA PISANI MONSELICE NOVENTA PADOVANA PADOVA BORGORICCO CAMPOSAMPIERO OSPEDALETTO EUGANEO ESTE BOVOLENTA PIOVE DI SACCO PADOVA PADOVA BRUGINE PIOVE DI SACCO PERNUMIA MONSELICE CADONEGHE PADOVA PIACENZA D'ADIGE ESTE CAMPO SAN MARTINO CITTADELLA PIAZZOLA SUL BRENTA CITTADELLA CAMPODARSEGO CAMPOSAMPIERO PIOMBINO DESE CAMPOSAMPIERO CAMPODORO CITTADELLA PIOVE DI SACCO PIOVE DI SACCO CAMPOSAMPIERO CAMPOSAMPIERO POLVERARA PIOVE DI SACCO CANDIANA CONSELVE PONSO ESTE CARCERI ESTE PONTE SAN NICOLO' PADOVA CARMIGNANO DI BRENTA CITTADELLA PONTELONGO PIOVE DI SACCO CARTURA CONSELVE POZZONOVO MONSELICE CASALE DI SCODOSIA ESTE ROVOLON PADOVA CASALSERUGO PADOVA RUBANO PADOVA CASTELBALDO ESTE SACCOLONGO PADOVA CERVARESE SANTA CROCE PADOVA SALETTO ESTE SAN GIORGIO DELLE CINTO EUGANEO ESTE CAMPOSAMPIERO PERTICHE CITTADELLA CITTADELLA SAN GIORGIO IN BOSCO CITTADELLA CODEVIGO PIOVE DI SACCO SAN MARTINO DI LUPARI CITTADELLA CONSELVE CONSELVE SAN PIETRO IN GU CITTADELLA CORREZZOLA PIOVE DI SACCO SAN PIETRO -

Discover Padua and Its Surroundings
2647_05_C415_PADOVA_GB 17-05-2006 10:36 Pagina A Realized with the contribution of www.turismopadova.it PADOVA (PADUA) Cittadella Stazione FS / Railway Station Porta Bassanese Tel. +39 049 8752077 - Fax +39 049 8755008 Tel. +39 049 9404485 - Fax +39 049 5972754 Galleria Pedrocchi Este Tel. +39 049 8767927 - Fax +39 049 8363316 Via G. Negri, 9 Piazza del Santo Tel. +39 0429 600462 - Fax +39 0429 611105 Tel. +39 049 8753087 (April-October) Monselice Via del Santuario, 2 Abano Terme Tel. +39 0429 783026 - Fax +39 0429 783026 Via P. d'Abano, 18 Tel. +39 049 8669055 - Fax +39 049 8669053 Montagnana Mon-Sat 8.30-13.00 / 14.30-19.00 Castel S. Zeno Sun 10.00-13.00 / 15.00-18.00 Tel. +39 0429 81320 - Fax +39 0429 81320 (sundays opening only during high season) Teolo Montegrotto Terme c/o Palazzetto dei Vicari Viale Stazione, 60 Tel. +39 049 9925680 - Fax +39 049 9900264 Tel. +39 049 8928311 - Fax +39 049 795276 Seasonal opening Mon-Sat 8.30-13.00 / 14.30-19.00 nd TREVISO 2 Sun 10.00-13.00 / 15.00-18.00 AIRPORT (sundays opening only during high season) MOTORWAY EXITS Battaglia Terme TOWNS Via Maggiore, 2 EUGANEAN HILLS Tel. +39 049 526909 - Fax +39 049 9101328 VENEZIA Seasonal opening AIRPORT DIRECTION TRIESTE MOTORWAY A4 Travelling to Padua: DIRECTION MILANO VERONA MOTORWAY A4 AIRPORT By Air: Venice, Marco Polo Airport (approx. 60 km. away) By Rail: Padua Train Station By Road: Motorway A13 Padua-Bologna: exit Padua Sud-Terme Euganee. Motorway A4 Venice-Milano: exit Padua Ovest, Padua Est MOTORWAY A13 DIRECTION BOLOGNA adova. -

Tomorrow Futurasun
Company Profile - EN Anticipate Tomorrow FuturaSun HEPPENHEIM (DE) Project Office offices and logistics FuturaSun was established in 2008 by a team of managers in Veneto, Italy’s hub of the photovoltaic industry. The headquarters are located in Cittadella, Padua Province. We have additional offices in the UK, Germany, Romania and BUCHAREST (RO) Headquarters in Cittadella, Sales Office Uganda. Padua Province Our main plants, with a 500 MW production capacity, are located in Taizhou and Maanshan, China. Here our containers are dispatched to customers all over the world. We also have another production plant located in Europe. Thanks to our warehouses in Italy and in the ports of BEDFORD (UK) Sales Office Rotterdam and Antwerp, we are able to guarantee an effective and rapid logistics service for all our customers in Europe. You can order full containers or even single pallets, because material is always available at our warehouses in Italy. CITTADELLA (IT) FuturaSun production plant Headquarters ROTTERDAM (NL) TAIZHOU (CN) Warehouse Manufacturing / Warehouse Logistics Service MAANSHAN (CN) Manufacturing / Warehouse How many modules are on a pallet? GALLIERA VENETA (IT) Warehouses in the ports of Rotterdam and Antwerp Warehouse 5 VIETNAM modules VENICE (IT) Manufacturing Warehouse 30 Double pallet modules with 65 modules PRODUCTION CAPACITY or single pallet EUROPE ASIA 30 with 30 modules modules Warehouse, north Italy 130 MW 500 MW 2 3 PATENTED 1 “SILK” STRINGER MACHINE We have filed the industrial patent for a new PV cell FuturaSun interconnection system. Technology and Production Since 2017 our Italian Research and Development department has been working on Silk, a new generation of high-efficiency 2 12-busbar crystalline silicon modules. -
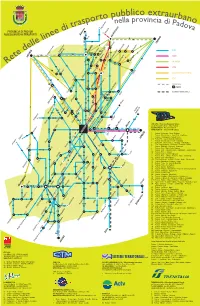
Mappa Linee Extraurbane
pubblico extraurb orto ella provincia ano rasp n di Pado i t Mussolente va PROVINCIA DI PADOVA d Bessica ASSESSORATO AI TRASPORTI e Bassano 24 21 3 e Castelfranco n Rosà 6 li 14 19 24 e TREVISO l 6 l Resana 3 e 24 Torreselle Levada Badoere SITA d 50 8 Carmignano 51 Cittadella49-50 Tombolo 2 S.Pietro in Gù di Brenta 74 29 e 14 14 ACTV S. Martino di L. t VICENZA 6 49 1 Loreggia Piombino Dese 60 e Loreggiola3 Onara LA MARCA 1 36 Trebaseleghe S. Ambrogio R 21 38 Abbazia 51 Fratte 40 74 29 19 17 CTM 2 50 Villalta Gazzo BONAVENTURA EXPRESS 1 Scorzè S. Giustina 36 Villa del Conte FTV 80 27 101 Piazzola 38 80 TRENITALIA 40 74 1 Camposampiero stazioni Arsego 29 S. Giorgio delle P. Lerino 101 Massanzago 40 Camisano 98/3 SISTEMI TERRITORIALI 2 8 98/2-98/3 98/3 Noale 38 21 Borgoricco41 Cavino 98/2 Villafranca 29 17 26 19 Montegaldella 40 74 Campodarsego Grisignano Mirano Zeminiana 4 36 103 26 Vigodarzere Limena Villanova Tessera 103 20 Veggiano Villaguattera Cadoneghe 60 Cervarese S. C. 31/5 37 Aeroporto 43 21 38 Mestrino 41 Vigonza 19 19-43 36 Barbarano 21/7 15/1 31/5 29 Pianiga Fossona Rubano 8 37 Bastia SITA S.P.A. • Direzione Regionale Veneto Dolo Mira-Mirano Mestre via del Pescarotto, 25/27 · 35131 Padova Teolo Ponte di Brenta 1 - 6 - 24 Tel. 049.8206811 · Fax 049.8206828 17-18-19-20 Vigonza-Pianiga 15/1 15/1 www.sitabus.it · [email protected] Sossano 53 43-103 6 PADOVA 73 62 1 Padova - Cavarzere - Adria - Codigoro Strà 2 Padova - Piove di Sacco - Correzzola - Cantarana 44 63 1-2-3-3/1-4-5-66-76 44 15/1 3 Padova - Campagnola - Piove di Sacco 102-103 Fossò VENEZIA Orgiano 17-18-102 3/1 Padova FS - Legnaro Agripolis Università Noventa P.na 70 Camponogara 4 Padova - Piove di Sacco - Civè - Cà Bianca Zovon 6 5 Padova FS - Piove di Sacco - Chioggia - Sottomarina Cologna 70 6 Este - Arquà Petrarca - Galzignano - Torreglia - Padova Saonara 102 Abano T. -

Mappa Delle Linee Extraurbane
MAPPA DELLE LINEE EXTRAURBANE Abano T. Piazza Fontana Padova Arcella Abano T. Villa Rigoni Padova Autostazione Abano Terme Stazione Padova Bassanello A B C D E F G H i Adria Padova Brusegana Aeroporto Marco Polo Padova Camin LEGENDA SCHEMA DELLE CORSIE DELL’AUTOSTAZIONE DI PADOVA INTERURBAN BUS TERMINAL Agna Padova Chiesanuova KEYS Viale della Pace, 1 - GPS: 45.4162111,11.8833668 e 029 Agripolis Campus Padova Corso Milano 1 e 036 1 Agugliaro Padova Guizza e 071 Capolinea e 038 Albignasego Padova Mandria Corsia 1 Corsia 3 Corsia 5 Corsia 7 Corsia 9 Corsia 11 First/last stop Platform 1 Platform 3 Platform 5 Platform 7 Platform 9 Platform 11 Corsia 13 Anguillara Veneta Padova Montà Linea 53E Platform 13 Arquà Petrarca Padova Ospedale Civile e 003 e 037 e 007 e 071 e 017 e A e071 Linea in transito e 041 Arre Padova Piazza Mazzini Running line e 006 e 033 e 073 e 018 e AT e 035 e 019 e ATL Arsego Padova Pontevigodarzere TSF e 060 Arzercavalli Padova Riviere Fermata di interscambio e 021 Bassano del Grappa e 040 e 020 e M Corsia 14 e 021 TSF Platform 14 Arzerello Padova S.Carlo Connection stop e 026 e T e 074 Arzergrande Padova Sacro Cuore Linea 101 e 031_6 e TL Padova Stanga Rosà e 001 VCE Linea feriale e festiva e 080 e 015 Badia Polesine Padova via Giotto Weekdays and Holydays e 002 e 010 Bus a lunga percorrenza Bagnoli di Sopra Padova via Toti 2 e 004 e 013 e 062 Long-distance buses 2 Baone Padova Voltabarozzo Linea prolungata Linea 208 e 005 e 016 Linea 80 e 063 Barbano Vicentino Padova Zona Industriale Cusinati Barbarano Palugana Extension -

ORARI SERVIZI AGGIUNTIVI PADOVA Aggiornati Al 14 Aprile 2021
ORARI SERVIZI AGGIUNTIVI PADOVA aggiornati al 14 Aprile 2021 Noi andiamo a scuola con Busitalia! LINEE POTENZIATE INDICE • La sicurezza viaggia con noi, e tu? pag. 3 • Urbano Padova e Linee dei Colli pag. 5 Urbano Padova pag. 5 Linee dei Colli pag. 8 • Legenda servizi aggiuntivi extraurbani pag. 9 • Elenco linee extraurbane potenziate pag. 10 • Extraurbano Padova pag. 12 • Informazioni e contatti pag. 49 2 LA SICUREZZA VIAGGIA CON NOI, E TU? INDOSSA CORRETTAMENTE LA NON METTERTI IN VIAGGIO SE HAI MASCHERINA A COPERTURA DI NASO E SINTOMI INFLUENZALI COME FEBBRE, BOCCA PRIMA DI SALIRE A BORDO E PER TOSSE, RAFFREDDORE. TUTTA LA DURATA DEL VIAGGIO. tolto un paio di parole SE IL BUS HA RAGGIUNTO LA PER VIAGGIARE, È NECESSARIO UN CAPIENZA MASSIMA DI PASSEGGERI REGOLARE TITOLO DI VIAGGIO. CONSENTITA, INDICATA NELLA ACQUISTALO PRIMA DI SALIRE A BORDO, XX SEGNALETICA SULLA PORTA DI SE IL BUS HA RAGGIUNTO LA CAPIENZA PRIVILEGIANDO I CANALI DIGITALI. MASSIMAACCESSO, DI PASSEGGERI ATTENDI IL CONSENTITA,BUS SUCCESSIVO. 50 50 3 LA SICUREZZA VIAGGIA CON NOI, E TU? RISPETTA LA DISTANZA DI SICUREZZA RICORDA DI IGIENIZZARE ALLE FERMATE E A BORDO. FREQUENTEMENTE LE MANI UTILIZZANDO GLI APPOSITI DISPENCER. NON AVVICINARTI ALL’AUTISTA PER CHIEDERE INFORMAZIONI E RICORDA NON UTILIZZARE I CHE LA VENDITA A BORDO DEI TITOLI POSTI CONTRASSEGNATI. DI VIAGGIO È SOSPESA. 4 URBANO PADOVA E LINEE DEI COLLI - orari servizi aggiuntivi URBANO PADOVA | LUNEDI' - VENERDI' Linea Descrizione Percorso Veletta a bordo bus Ora P Ora A U03 Lion FS Severi U03 FERROVIA SEVERI 07:05 08:00 U03 Ferrovia Lion U03 LION 12:50 13:35 U04 Cadoneghe P.Valle U04 FERROVIA P.VALLE 07:20 08:00 U05 Voltabrusegana FS Severi U05 FERROVIA SEVERI 07:15 08:00 U06 Terranegra Lagrange via Sorio U06 LAGRANGE (PER VIA SORIO) 07:14 08:04 U06 Ferrovia Lagrange via Sorio U06 LAGRANGE (PER VIA SORIO) 07:24 07:44 U06 Ferrovia duca degli Abruzzi U06 B. -

Comune Di Cittadella
COMUNE DI CITTADELLA PROVINCIA DI PADOVA SERVIZI ECONOMICO FINANZIARI RAGIONERIA DETERMINAZIONE DIRIGENZIALE N. 1236 / 2015 del 23/12/2015 OGGETTO: APPROVAZIONE ELENCO VIOLAZIONI AL CODICE DELLA STRADA ED ENTRATE PATRIMONIALI NON VERSATE DA SOTTOPORRE A RISCOSSIONE COATTIVA MEDIANTE INGIUNZIONE EX R.D. N. 639_1910 - PAGAMENTO COMPENSO ALL'ABACO S.P.A. IL DIRIGENTE/IL RESPONSABILE DI SERVIZIO Richiamate: - la determinazione 9.6.2014, n. 96, a firma del Dirigente del Settore Economico Finanziario di affidamento in concessione del servizio di riscossione coattiva delle entrate tributarie e patrimoniali dell’Ente alla ditta ABACO S.p.A., via F.lli Cervi, 6, Padova C.F. 02391510266; - la convenzione n. 3462 di repertorio, in data 12.9.2014, relativa al contratto d’appalto del servizio anzidetto sottoscritto dalla ditta ABACO S.p.A., via F.lli Cervi, 6, Padova, C.F. 02391510266; Visto che il Distretto di Polizia Locale PD1A ha predisposto l’elenco degli utenti che non hanno versato le sanzioni per le violazioni al Codice della strada (Allegato A) da sottoporre a riscossione coattiva a mezzo ingiunzione ex R.D. n. 639/1910; Visto inoltre che l’Ufficio Tributi ha predisposto l’elenco degli insolventi per mancato versamento oneri di urbanizzazione ed altre entrate patrimoniali da sottoporre a riscossione coattiva tramite ingiunzione ex R.D. n. 639/1910 (Allegato B); Visto che la riscossione coattiva dei crediti derivanti dall’I.C.I., dalle sanzioni per le violazioni al Codice della strada e dalle altre entrate patrimoniali è affidata per il periodo 1.1.2011/31.12.2016 alla ditta ABACO S.p.A; Preso atto che l’importo da recuperare mediante riscossione coattiva ammonta ad € 1.217.953,00 per quanto riguarda i crediti derivanti dalle sanzioni per le violazioni al Codice della strada ed ad € 18.217,64 per quanto riguarda gli oneri di urbanizzazione ed altre entrate patrimoniali; Preso atto che il compenso e le spese di notifica per la riscossione coattiva dei succitati crediti da corrispondere alla ditta ABACO S.p.A. -

Trebaseleghe
CALENDARIO 2021 La Natura Insegna Scuola | Educazione | Ambiente | Sostenibilità GUIDA 2021 PER LA RACCOLTA DIFFERENZIATA ZONA A B Comune di TREBASELEGHE SERVIZIO CLIENTI SERVIZIO RIFIUTI DAL LUNEDÌ AL VENERDÌ 8.00 - 20.00 (servizio disponibile nei giorni lavorativi) CONTATTACI PER: 800 247842 • Informazioni sul servizio di raccolta e sulla corretta differenziazione dei rifiuti NUMERO VERDE • Segnalare la mancata raccolta di qualche tipo di rifiuto,dalle ore 14 del giorno di raccolta previsto ed entro 2 giorni, specificando tipologia di Tenere a portata di mano rifiuto e data della mancata raccolta codice anagrafico o codice • Informazioni sulla tariffa rifiuti e chiarimenti sulla bolletta servizio dell’utenza interessata • Prenotare il ritiro a domicilio dei rifiuti Ingombranti e RAEE, Inerti, Toner (solo per utenze non domestiche) ALTRI CONTATTI E-mail Posta ordinaria Sito internet [email protected] Etra Spa www.etraspa.it [email protected] via del Telarolo, 9 35013 Cittadella (PD) SPORTELLI ASIAGO BASSANO DEL GRAPPA via F.lli Rigoni Guido e Vasco, 19 via C. Colombo, 96 36012 Asiago (VI) 36061 Bassano del Grappa (VI) dal lunedì al venerdì 8.30 - 13.00 lunedì, mercoledì, giovedì e venerdì 8.30 - 13.00; 14.30 - 17.00 NOVE martedì (orario continuato) 8.30 - 17.00 via Padre Roberto, 50 - 36055 Nove (VI) venerdì 8.30 - 13.00 CITTADELLA presso il Centro commerciale “Le Torri” SAN PIETRO IN GU via Copernico, 2A - 35013 Cittadella (PD) presso il Centro culturale comunale dal lunedì al venerdì piazza Prandina, 34 8.30 - 13.00; 14.30 - -

Tra Cittadella E Piazzola Sul Brenta
TRA CITTADELLA E PIAZZOLA SUL BRENTA Tra le molte iniziative che i Girasoli promuovono con lo scopo di trascorrere i fine settimana all’aria aperta è stata inserita la gita denominata tra Cittadella e Piazzola, due località con il passato illustre che anche il giorno d’oggi sono piene di storia grazie ai monumenti e ville che sono rimaste intatte nel tempo. Da questo presupposto la nostra socia e “consigliera” del Club signora Roberta Rossi, coadiuvata dal marito Franco, ha pensato di organizzare una gita in queste due belle cittadine, per portare i nostri soci a visitare luoghi del passato ma con un presente molto vivo. La gita è cominciata venerdì 4 aprile con l’arrivo di un nutrito numero di equipaggi (quasi 30) nel parcheggio di Villa Rina messo a disposizione dall’Amministrazione Comunale di Cittadella alla quale va tutta la nostra riconoscenza. La gita è proseguita sabato mattina, 5 aprile, per la visita guidata alla cinta muraria, da poco ristrutturata e rimessa a nuovo, del Duomo e del Teatro sociale. Causa i molti partecipanti per le visite sono stati creati due gruppi. Interessante sotto l’aspetto storico la visita a Cittadella e più precisamente alle mura cittadine di origine medievale che si inseriscono, come periodo, a quelle costruite ad Asolo, Bassano del Grappa, Marostica e Castelfranco Veneto. Cittadella è stata costruita nel 1220, quando la città di Padova decise di creare, ex novo, nel settore centrale della pianura un luogo fortificato per controllare le zone di confine contro le città di Treviso e Vicenza. Degno di menzione è anche il teatro Comunale che sorge vicino a Porta Vicenza e progettato nel 1817.