Bro Taf Formulary Since the Most Recent Version of the Formulary Was Posted on the WMIC Website
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

By in Vivo's Biopharma, Medtech and Diagnostics Teams
invivo.pharmaintelligence.informa.com JANUARY 2018 Invol. 36 ❚ no. 01 Vivopharma intelligence ❚ informa 2018 OUTLOOK By In Vivo’s Biopharma, Medtech and Diagnostics Teams PAGE LEFT BLANK INTENTIONALLY invivo.pharmaintelligence.informa.com STRATEGIC INSIGHTS FOR LIFE SCIENCES DECISION-MAKERS CONTENTS ❚ In Vivo Pharma intelligence | January 2018 BIOPHARMA MEDTECH 2018 DIAGNOSTICS OUTLOOK 12 22 28 Biopharma 2018: Medtech 2018: Diagnostics 2018: Is There Still A Place For Pharma The Place For Innovation Steady Progress And In The New Health Care As Value-based Health Care The Big Get Bigger Economy? Gains Momentum MARK RATNER WILLIAM LOONEY ASHLEY YEO If the beginning of 2017 was marked 2018 will be a time of transition in health 2017 was a watershed year in many by doubts around whether and how care, when biopharma’s counterparts respects, politically, economically the FDA would act with respect to in adjacent industry segments scale up and commercially for many players complex diagnostics, we enter 2018 in a radical redesign of their traditional in the medtech field. Where will the feeling that slow-moving vessel may business models. Biopharma is not opportunities lie in 2018? Will finally be turning. moving as quickly, and it confronts a breakthrough medtech innovation still strategic dilemma on how to address the have a place among providers often prospect of a much more powerful set of riding on fumes when it comes to 36 rivals in the ongoing battle to own the budgets, and is it all as bad as some patient experience in medicine. would make out? Thirty-five Years Covering Health Care: The More Things Change… 30 PETER CHARLISH A Virtuous Cycle: What The The health care industry has come a Immuno-Oncology Revolution long way in the past 35 years, although Means For Other Disease Areas in some areas very little has changed. -

Bonviva, INN- Ibandronic Acid
European Medicines Agency London, 1 September 2005 Doc.Ref.: EMEA/278873/2005 Bonviva International Nonproprietary Name: Ibandronic acid Following the procedure EMEA/H/C/501/X/01 7 Westferry Circus, Canary Wharf, London E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86 68 E-mail: [email protected] http://www.emea.eu.int EMEA 2005 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged 1 SCIENTIFIC DISCUSSION 1.1 Introduction and rationale The MAH submitted an extension application under Annex II, point 2 iii to Commission Regulation (EC) No 1085/2003 to request the approval of a 150 mg tablet as a monthly dosing regimen of ibandronate only for the indication of “treatment of osteoporosis in postmenopausal women, in order to reduce the risk of vertebral fractures”. This claim is based on the results of a phase III study MOBILE (BM16549) comparing 100 and 150 mg once monthly to 2.5 mg once daily. Due to the inconveniences associated with intake of oral bisphosphonates (i.e. fasting conditions, frequent upper gastrointestinal intolerance) that may result in poor compliance, it was considered desirable to develop a more convenient drug formulation. Hence a 150 mg once monthly oral regimen is expected to offer greater convenience to postmenopausal women when compared to the currently approved 2.5mg once daily tablet. The development programme No new pre-clinical pharmacodynamic and pharmacokinetic studies have been performed in addition to those included in the previous submission for ibandronate 2.5 mg daily oral tablets. -

CCA Senior Care Options Formulary
Commonwealth Care Alliance Senior Care Option HMO SNP 2021 List of Covered Drugs Formulary 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN This formulary was updated on 08/01/2021. For more recent information or other questions, please contact Senior Care Options Program (HMO SNP) Member Services, at 1-866-610-2273 or, for TTY users, 711, 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthcaresco.org. HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Senior Care Options Program (HMO SNP) 2021 Formulary (List of Covered Drugs) PLEASE READ: THIS DO CUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN HPMS Approved Formulary File Submission ID 00021589, Version Number 13 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us”, or “our,” it means Commonwealth Care Alliance. When it refers to “plan” or “our plan,” it means 2021 Senior Care Options Program. This document includes list of the drugs (formulary) for our plan which is current as of 08/01/2021. This formulary document applies to all SCO members. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. Benefits, formulary, pharmacy n etwork, and/or copayments/coinsurance may change on January 1, 2022, and from time to time during the year. -

Bisphosphonate Use in Patients with Breast Cancer
Bisphosphonate use in patients with breast cancer Information for patients, relatives and carers For more information, please contact: Medicines Information Email: [email protected] York Hospital Medicines Information Tel: 01904 725960 Page 2 Contents Page Why have I been given this leaflet? ............................... 4 How is the treatment given? .......................................... 4 How long will I need to take the treatment for? .............. 6 Can I take other medicines at the same time? ............... 6 Who will prescribe the medication? ............................... 7 What if I am already on bisphosphonate treatment? ...... 7 Bisphosphonates are not licensed for reducing the risk of breast cancer recurrence. What does this mean? ...... 8 Are there any side effects? ............................................ 9 What is osteonecrosis? ............................................... 10 Are there any signs and symptoms I should look out for during treatment? ........................................................ 11 How can I decrease the risk of developing osteonecrosis of the jaw? ................................................................... 12 What do I need to do before starting treatment? .......... 13 Will I require any monitoring, or blood tests? ............... 14 Should I stop taking the bisphosphonate? ................... 14 Tell us what you think of this leaflet ............................. 15 Teaching, training and research ................................... 15 Patient Advice and Liaison Service (PALS) .................. 15 Page 3 Why have I been given this leaflet? You have been given this leaflet because you are going to be treated with a bisphosphonate medicine (zoledronic acid and/or ibandronic acid). In post-menopausal women, with early breast cancer, these drugs have been shown to reduce the risk of the disease recurring and increase length of life. How is the treatment given? The treatment will usually be provided as an oral tablet of ibandronic acid (one 50mg tablet taken once daily). -

Appendix 1 Definitions of Words and Phrases Used in the Act And
Appendix 1 Definitions of Words and Phrases Used in the Act and Subordinate Legislation The persons responsible for drafting a piece of legislation aim to achieve clarity and avoid ambiguity in the document. However, most words have shades of meaning and the interpretation of the statute or instrument becomes difficult for those who have to obey or enforce it, especially when such persons are not law yers. In the last analysis, it is a judge who has to decide whether a particular set of circumstances fall within or outside a given set of words, but this may be too late for the citizen who may have done utmost to keep within the law. It should, however, be pointed out that government departments and others who have a duty to enforce a piece of legislation are usually willing to advise members of the public as to its meaning and effects. In order to help those who have to obey or enforce the law an 'interpretation' section, regulation or rule containing a number of technical terms used in the document is often included. In the Medicines Act, sections 130,131 and 132 are devoted to this purpose. However, many other terms are defined in other sec tions and are sometimes less easy to locate. Furthermore, numerous statutory instruments also define various words and phrases and these definitions are not always identical with those used in the Act. To facilitate reference to these defi nitions, they have been collected together and printed alphabetically with the exception of 'medicinal product' and 'wholesale' and 'retail' sale which, in view of their importance, are treated separately. -
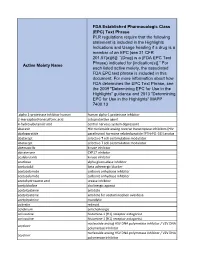
Active Moiety Name FDA Established Pharmacologic Class (EPC) Text
FDA Established Pharmacologic Class (EPC) Text Phrase PLR regulations require that the following statement is included in the Highlights Indications and Usage heading if a drug is a member of an EPC [see 21 CFR 201.57(a)(6)]: “(Drug) is a (FDA EPC Text Phrase) indicated for [indication(s)].” For Active Moiety Name each listed active moiety, the associated FDA EPC text phrase is included in this document. For more information about how FDA determines the EPC Text Phrase, see the 2009 "Determining EPC for Use in the Highlights" guidance and 2013 "Determining EPC for Use in the Highlights" MAPP 7400.13. .alpha. -

Illness Code A
Long Term Illness (LTI) Scheme: Reference Guide as to what is covered in the Health Service Executive for each of the 16 scheduled LTI illnesses. (January 2021) Illness Code C: Cerebral Palsy Antibiotics 1. Doxycycline J01AA02 2. Lymecycline J01AA04 3. Minocycline J01AA08 4. Ampicillin J01CA01 5. Amoxicillin J01CA04 6. Azlocillin J01CA09 7. Benzylpenicillin J01CE01 8. Phenoxymethylpenicillin J01CE02 9. Flucloxacillin J01CF05 10. Amoxicillin & Enzyme Inhibitor J01CR02 11. Cefalexin J01DB01 12. Cefuroxime J01DC02 13. Cefaclor J01DC04 14. Ceftazidime J01DD02 15. Ceftriaxone J01DD04 16. Cefixime J01DD08 17. Cefpodoxime J01DD13 18. Trimethoprim J01EA01 19. Sulfamethoxazole & Trimethoprim J01EE01 20. Erythromycin J01FA01 21. Clarithromycin J01FA09 22. Azithromycin J01FA10 23. Clindamycin J01FF01 24. Gentamicin J01GB03 25. Amikacin J01GB06 26. Ofloxacin J01MA01 27. Ciprofloxacin J01MA02 28. Levofloxacin J01MA12 29. Moxifloxacin J01MA14 30. Vancomycin J01XA01 31. Teicoplanin J01XA02 32. Colistin J01XB01 33. Fusidic Acid J01XC01 34. Nitrofurantoin J01XE01 Muscle relaxants 1. Baclofen M03BX01 2. Tizanidine M03BX02 3. Dantrolene M03CA01 Anticonvulsants (including Benzodiazepines used for this purpose) 1. Phenobarbital N03AA02 2. Primidone N03AA03 page 1 3. Phenytoin N03AB02 4. Ethosuximide N03AD01 5. Clonazepam N03AE01 6. Carbamazepine N03AF01 7. Oxcarbazepine N03AF02 8. Rufinamide N03AF03 9. Eslicarbazepine N03AF04 10. Valproic Acid N03AG01 11. Vigabatrin N03AG04 12. Tiagabine N03AG06 13. Lamotrigine N03AX09 14. Topiramate N03AX11 15. Gabapentin N03AX12 16. Levetiracetam N03AX14 17. Zonisamide N03AX15 18. Pregabalin N03AX16 19. Lacosamide N03AX18 20. Retigabine N03AX21 21. Perampanel N03AX22 22. Diazepam (Rectal) N05BA01 23. Clobazam N05BA09 24. Midazolam N05CD08 25. Acetazolamide S01EC01 Anxiolytics 1. Diazepam N05BA01 2. Chlordiazepoxide N05BA02 3. Lorazepam N05BA06 4. Bromazepam N05BA08 5. Clobazam N05BA09 6. Prazepam N05BA11 7. Alprazolam N05BA12 Hypnotics 8. -

Therapeutic Consequences of the Drug Lag 93 X Recent Developments, 1972 to 1974 ...•
REGULATION AND DRUG DEVELOPMENT William M. Wardell and Louis Lasagna American Enterprise Institute for Public Policy Research Washington. D. C. CONTENTS INTRODUCTION .. ................... 1 PART ONE Science. Government, and the Current State of Affairs I PRE-1962 PRACTICES: THE EVOLUTION OF CON- TROLS OVER THERAPEUTIC DRUGS 5 II POST-1962 GUIDELINES AND PRACTICES 19 III THE NATURE OF EVIDENCE 27 IV WEIGHING THE EVIDENCE 37 V ECONOMICS AND DRUG DEVELOPMENT 45 PART TWO Lessons from Other Countries VI APPROACHES TO INTERNATIONAL COMPARISONS 51 VII PATTERNS OF INTRODUCTION OF NEW DRUGS IN BRITAIN AND THE UNITED STATES 55 VIII BRITISH USAGE AND AMERICAN AWARENESS OF SOME NEW DRUGS 79 IX THERAPEUTIC CONSEQUENCES OF THE DRUG LAG 93 X RECENT DEVELOPMENTS, 1972 TO 1974 ...•....... 109 PART THREE The Wider Issues XI THE INTENT OF CONGRESS AND THE NEEDS OF PATIENTS 127 XII THE RUBRICS REEXAMINED 135 XIII SUGGESTIONS FOR IMPROVEMENT. ............. .. 143 XIV CONCLUSIONS.................................... 161 APPENDIX: Definition of "Substantial Evidence" 167 NOTES 171 INTRODUCTION In the late 1950s the Congress of the United States began holding subcommittee hearings on various matters pertaining to the pharma ceutical industry. Two of the most vigorous and sensational were those chaired by Congressman Blatnik and by Senator Kefauver. They were highly critical of the quality of drug advertising, theevi dence for effectiveness of marketed pharmaceuticals, the allegedly monopolistic nature of the drug industry, and the price of medicines. The drug industry, accustomed to respect and gratitude from physicians and the public, was suddenly confronted with hostile, sensational newspaper headlines and stories and a rising wave of angry criticism. -
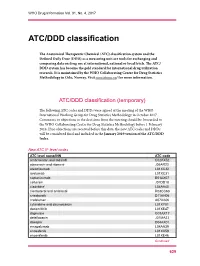
ATC/DDD Classification
WHO Drug Information Vol. 31, No. 4, 2017 ATC/DDD classification The Anatomical Therapeutic Chemical (ATC) classification system and the Defined Daily Dose (DDD) as a measuring unit are tools for exchanging and comparing data on drug use at international, national or local levels. The ATC/ DDD system has become the gold standard for international drug utilization research. It is maintained by the WHO Collaborating Centre for Drug Statistics Methodology in Oslo, Norway. Visit www.whocc.no/ for more information. ATC/DDD classification (temporary) The following ATC codes and DDDs were agreed at the meeting of the WHO International Working Group for Drug Statistics Methodology in October 2017. Comments or objections to the decisions from the meeting should be forwarded to the WHO Collaborating Centre for Drug Statistics Methodology before 1 February 2018. If no objections are received before this date, the new ATC codes and DDDs will be considered final and included in the January 2019 version of the ATC/DDD Index. New ATC 5th level codes ATC level name/INN ATC code ambrisentan and tadalafil C02KX52 atazanavir and ritonavir J05AR23 atezolizumab L01XC32 avelumab L01XC31 caplacizumab B01AX07 cefteram J01DD18 cladribine1 L04AA40 clenbuterol and ambroxol R03CC63 crisaborole D11AH06 crofelemer A07XA06 cytarabine and daunorubicin L01XY01 dacomitinib L01XE47 dapivirine G01AX17 delafloxacin J01MA23 doxepin D04AX01 emapalumab L04AA39 enasidenib L01XX59 encorafenib L01XE46 Continued 629 ATC/DDD classification WHO Drug Information Vol. 31, No. 4, 2017 -

An Expert System for Development of Powder Filled Hard Gelatin Capsule Formulations
An Expert System For Development of Powder Filled Hard Gelatin Capsule Formulations Thesis submitted to the University of London Faculty of Medicine for THE DEGREE OF DOCTOR OF PHILOSOPHY ky Samantha Miu Ha Lai B.Pharm., M.R.Pharm.S. September 1996 The School of Pharmacy University of London Brunswick Square London WCIN lAX ProQuest Number: 10104253 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10104253 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Dedicated to my Father ACKNOWLEDGMENT I would like to dedicate this thesis to my father, without whom I would not be able to achieve this PhD. My sister, Esther and my boyfriend, Eric were also very understanding and have been most supportive throughout my ‘ups and downs’. The guidance and supervision provided by my supervisor. Professor J M Newton and also by Dr F Podczeck also made this PhD possible. Special thanks must go to Professor Newton for being so incredibly patient and spending so much time with me on top of his busy schedule. -

Position Paper American Association of Oral and Maxillofacial Surgeons
saving faces|changing lives® Position Paper American Association of Oral and Maxillofacial Surgeons Medication-Related Osteonecrosis of the Jaw—2014 Update Special Committee on Medication- Introduction Related Osteonecrosis of the Jaws: The Special Committee recommends changing the Salvatore L. Ruggiero, DMD, MD, Clinical nomenclature of bisphosphonate-related osteonecrosis of Professor, Division of Oral and Maxillofacial the jaw (BRONJ). The Special Committee favors the term Surgery, Stony Brook School of Dental Medicine, medication-related osteonecrosis of the jaw (MRONJ). Hofstra North Shore-LIJ School of Medicine, The change is justifed to accommodate the growing New York Center for Orthognathic and Maxillo- number of osteonecrosis cases involving the maxilla and facial Surgery, Lake Success, NY mandible associated with other antiresorptive (denosumab) and antiangiogenic therapies. Thomas B. Dodson, DMD, MPH, Professor and Chair, Associate Dean for Hospital Affairs, MRONJ adversely affects the quality of life, produc- University of Washington School of Dentistry, ing signifcant morbidity. Strategies for management of Department of Oral and Maxillofacial Surgery, patients with, or at risk for, MRONJ were set forth in the Seattle, WA American Association of Oral and Maxillofacial Sur- geons (AAOMS) updated Position Paper on Bisphospho- John Fantasia, DDS, Chief, Division of Oral nate-Related Osteonecrosis of the Jaws and approved by Pathology, Hofstra North Shore-LIJ School of the Board of Trustees in 2009.1 The Position Paper was Medicine , New Hyde Park, NY developed by a Special Committee appointed by the Board Reginald Goodday, Professor, Department of Oral and composed of clinicians with extensive experience in and Maxillofacial Sciences, Dalhousie University, caring for these patients and basic science researchers. -

Recommendations from York and Scarborough Medicines Commissioning Committee September 2017
Recommendations from York and Scarborough Medicines Commissioning Committee September 2017 Drug name Indication Recommendation, rationale and place in therapy RAG status Potential full year cost impact 1 TA463: Cabozantinib for previously Recommended as an option for treating advanced Red No cost impact to CCGs as NHS treated advanced renal cell renal cell carcinoma in adults after VEGF-targeted England commissioned. carcinoma therapy, only if company provides it with discount agreed in the PAS. 2 TA464: Bisphosphonates for Oral bisphosphonates (alendronic acid, ibandronic acid All drugs Low/no cost impact expected. It is not treating osteoporosis and risedronate sodium) are recommended as options listed in the expected that practice will change for treating osteoporosis in adults only if: guidance are substantially as a result of this the person is eligible for risk assessment as already on guidance. defined in NICE's guideline on osteoporosis and formulary as green (oral) the 10‑year probability of osteoporotic fragility or red (IV) for fracture is at least 1%. osteoporosis Intravenous bisphosphonates (ibandronic acid and zoledronic acid) are recommended as options for treating osteoporosis in adults only if: the person is eligible for risk assessment as defined in NICE's guideline on osteoporosis and the 10‑year probability of osteoporotic fragility fracture is at least 10% or the 10‑year probability of osteoporotic fragility fracture is at least 1% and the person has difficulty taking oral bisphosphonates (alendronic acid, ibandronic acid or risedronate sodium) or these drugs are contraindicated or not tolerated. 3 TA465: Olaratumab in combination Olaratumab, in combination with doxorubicin, is Red No cost impact to CCGs as NHS with doxorubicin for treating recommended for use within the Cancer Drugs Fund England commissioned.