100 People Who Changed History and the World
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Resource Collection for High Ability Secondary Learners 2011
Resource Collection for High Ability Secondary Learners Office of Gifted Education Montgomery County Public Schools 2011 - 2012 Table of Contents 2011 – 2012 Materials for High Ability Secondary Students How to Order .................................................................................................................................. 3 Professional Resources for Teachers .............................................................................................. 4 Differentiation ............................................................................................................................. 4 Assessment .................................................................................................................................. 5 Learning Styles and Multiple Intelligences ................................................................................ 6 Curriculum, Strategies and Techniques ...................................................................................... 7 Miscellaneous ............................................................................................................................. 9 English ...................................................................................................................................... 10 Mathematics .............................................................................................................................. 13 History...................................................................................................................................... -

19680011654.Pdf
NASA TECHNICAL NOTE -NASA TN-- D-4449 c. 1 o* w d nP z c e r/l e z LOAN COPY: RETURN TO AFWL IWLIL-2) KIRTLAND AFB, N MU( NATIONAL AERONAUTICS AND SPACE ADMINISTRATION WASHINGTON, D. C. APRIL 1968 ~ TECH LIBRARY KAFB, NM Iillllllllllllll llllllllll lllllllllllllllll NAVIGATOR PERFORMANCE STUDIES FOR SPACE NAVIGATION USING THE NASA CV-990 RESEARCH AIRCRAFT By Richard A. Acken and Donald W. Smith Ames Research Center Moffett Field, Calif. NATIONAL AERONAUTICS AND SPACE ADMINISTRATION - For sole by the Clearinghouse for Federal Scientific and Technical Information Springfield, Virginia 22151 - CFSTI price $3.00 NAVIGATOR PERFORMANCE STUDES FOR SPACE NAVIGATION USING THE NASA CV-990 RESEARCH AIRCRAFT By Richard A. Acken and Donald W. Smith Ames Research Center Manually operated hand-held sextants are being studied at Ames Research Center to determine whether they are sufficiently accurate for midcourse navigation phases of manned space flight. Studies carried out on the ground have been extended by using the NASA CV-990 aircraft to provide sighting con- ditions closely simulating those in spacecraft and to investigate firther the measurement error due to lunar irradiance. The results of approximately 1200 measurements made during nine flights confirm results of simulator and ground- based studies which indicate that, with a hand-held sextant, an astronaut can be expected to make navigational measurements with errors having a standard deviation of approximately 210 arc seconds. A value for moon irradiance effect of approximately 25 arc seconds was established for the conditions of the experiment using a hand-held sextant. INTRODUCTION The manually operated hand-held sextant has provided marine navigators with a compact, lightweight, easily operated instrument for centuries. -

SPRING Nav-06.Indd
SPRING 2006 AUXILIARY LAYS WREATH VOL. 33, NO. 1 AT NATIONAL CEMETERY ® s Last November, the U.S. Coast Guard Auxiliary was granted the rare privilege of laying a wreath at the Tomb of the Unknowns in Arlington National Cemetery in Virginia. The honor of actually laying the wreath was bestowed upon Joe Lifesaver Volunteer America’s Stern, IPDCP-11 D1SR, (second from left), who received permission from Arlington National Cemetary to perform the ceremony. A U.S. Marine Corps veteran and former Korean War POW, Stern commented, “This was one of the greatest honors I ever had.” Joining him at the ceremony are Gail Venezio, DDO; Tom Venezio, VCO; and Manuel Padilla, FC 11-11 all D1SR. Photo by Burt Hurvich, D-AA and DSO-PA 1SR. AMERICA’S Coast Guard Auxiliary Association, Inc. NON-PROFIT WATERWAY WATCH The Auxiliary Center U.S. POSTAGE PAID PERMIT NO. 842 9449 Watson Industrial Park JACKSONVILLE, FL. IN ACTION! St. Louis, MO 63126 Address service requested AST G CO U . S E M P E R A AUXILIARY GUARD COAST STATES UNITED S R . D U S P A A R A T U U Y XILIA R Spring 2006 Navigator 1 Auxiliary Artist Accepted AST G CO U . S E M P E R A S R . D U S P A A R A T U U Y XILIA R Contents SPRING 2006 VOL. 33, NO. 1 3 Rare Medal awarded to NY Auxiliarist 5 Dogs Learning to Fly 6 COMO Seibert 8 NACON 2006 is set 11 Interpreter asked to help on Midway 12 Color Guard launches dedication ceremonies 37 2006-07 13 U.S.-Canada RBS Initiatives EDITION 14 In Memoriam DEADLINES FALL 17 Auxiliary Wins NWSC and AFRAS awards AUGUST 15 18 USCG Rear Admiral and CGAUX WINTER Division Captain Cross Paths NOVEMBER 15 SPRING 20 Auxiliary Training Saves the Day FEBRUARY 15, 2007 23 New Requirements Cape Cod Jayhawk About to Embark on Katrina Rescue. -

The Drink Tank Sixth Annual Giant Sized [email protected]: James Bacon & Chris Garcia
The Drink Tank Sixth Annual Giant Sized Annual [email protected] Editors: James Bacon & Chris Garcia A Noise from the Wind Stephen Baxter had got me through the what he’ll be doing. I first heard of Stephen Baxter from Jay night. So, this is the least Giant Giant Sized Crasdan. It was a night like any other, sitting in I remember reading Ring that next Annual of The Drink Tank, but still, I love it! a room with a mostly naked former ballerina afternoon when I should have been at class. I Dedicated to Mr. Stephen Baxter. It won’t cover who was in the middle of what was probably finished it in less than 24 hours and it was such everything, but it’s a look at Baxter’s oevre and her fifth overdose in as many months. This was a blast. I wasn’t the big fan at that moment, the effect he’s had on his readers. I want to what we were dealing with on a daily basis back though I loved the novel. I had to reread it, thank Claire Brialey, M Crasdan, Jay Crasdan, then. SaBean had been at it again, and this time, and then grabbed a copy of Anti-Ice a couple Liam Proven, James Bacon, Rick and Elsa for it was up to me and Jay to clean up the mess. of days later. Perhaps difficult times made Ring everything! I had a blast with this one! Luckily, we were practiced by this point. Bottles into an excellent escape from the moment, and of water, damp washcloths, the 9 and the first something like a month later I got into it again, 1 dialed just in case things took a turn for the and then it hit. -

Communications-Mathematics and Applied Mathematics/Download/8110
A Mathematician's Journey to the Edge of the Universe "The only true wisdom is in knowing you know nothing." ― Socrates Manjunath.R #16/1, 8th Main Road, Shivanagar, Rajajinagar, Bangalore560010, Karnataka, India *Corresponding Author Email: [email protected] *Website: http://www.myw3schools.com/ A Mathematician's Journey to the Edge of the Universe What’s the Ultimate Question? Since the dawn of the history of science from Copernicus (who took the details of Ptolemy, and found a way to look at the same construction from a slightly different perspective and discover that the Earth is not the center of the universe) and Galileo to the present, we (a hoard of talking monkeys who's consciousness is from a collection of connected neurons − hammering away on typewriters and by pure chance eventually ranging the values for the (fundamental) numbers that would allow the development of any form of intelligent life) have gazed at the stars and attempted to chart the heavens and still discovering the fundamental laws of nature often get asked: What is Dark Matter? ... What is Dark Energy? ... What Came Before the Big Bang? ... What's Inside a Black Hole? ... Will the universe continue expanding? Will it just stop or even begin to contract? Are We Alone? Beginning at Stonehenge and ending with the current crisis in String Theory, the story of this eternal question to uncover the mysteries of the universe describes a narrative that includes some of the greatest discoveries of all time and leading personalities, including Aristotle, Johannes Kepler, and Isaac Newton, and the rise to the modern era of Einstein, Eddington, and Hawking. -

A Characteristic of a Navigator's Situation Awareness for Crossing Ships
the International Journal Volume 11 on Marine Navigation Number 2 http://www.transnav.eu and Safety of Sea Transportation June 2017 DOI: 10.12716/1001.11.02.12 A Characteristic of a Navigator's Situation Awareness for Crossing Ships C. Nishizaki, T. Takemoto & Y. Kunieda Tokyo University of Marine Science and Technology, Tokyo, Japan ABSTRACT: Many ship collisions have been caused by a navigator’s error in the situation awareness (SA) of the navigator. In congested sea areas, navigators classify ships on the basis of different priority levels. For safety measures against ship collision, it is imperative for navigators to recognize the ships with high priority levels. In previous study, navigators’ SA was measured in a ship maneuvering simulator using the Situation Awareness Global Assessment Technique (SAGAT). From the results of the previous study, we proposed a new risk category, named as “attention area,” that covers ships with high priority level in the SA of navigators. However, the extent of data for navigators’ SA was limited. Therefore, the purpose of this study is to confirm the validity of the category using additional data of navigators SA. In this study, the validity of the proposed category was confirmed, and a limit line surrounding ships with high priority levels was identified. In addition, it was evident that the category was able to detect ships with high priority level around the time when the collision avoidance was performed. 1 INTRODUCTION maneuvering simulator, the possibility that trainees’ navigation skills could be measured by SAGAT was Many marine accidents are caused by errors on the indicated (Okazaki & Ohya 2012). -

Stone Spring Free
FREE STONE SPRING PDF Stephen Baxter | 528 pages | 10 Feb 2011 | Orion Publishing Co | 9780575089204 | English | London, United Kingdom Stone Spring - Wikipedia Alternate history at its most mindblowing-from the national bestselling author of Flood and Ark. Ten thousand years ago, a vast and fertile plain Stone Spring linking the British Stone Spring to Europe. Home to a tribe of simple hunter-gatherers, Northland teems with nature's bounty, but is also Stone Spring to its whims. Fourteen-year-old Ana calls Northland home, but her world is changing. The air is warming, the ice is melting, and the seas are rising. Then Ana meets a traveler from a far-dista. Then Ana meets a traveler from a far-distant city called Jericho-a city that is protected by a wall. And she starts to imagine the impossible Praise for Stephen Baxter and Stone Spring. Stephen Baxter Stone Spring born in Liverpool, England, in He holds degrees in mathematics, from Cambridge University; engineering, from Southampton University; and business administration, from Henley Management College. His first professionally published short story appeared in He has also published over sf short stories, several of which have won prizes. Goodreads helps you keep track Stone Spring books you want to read. Want to Read saving…. Want to Read Currently Reading Read. Other editions. Enlarge cover. Error rating book. Refresh and try again. Open Preview See a Problem? Details if other :. Thanks for telling us about the problem. Return to Book Page. Preview — Stone Spring by Stephen Baxter. Stone Spring Northland 1 by Stephen Baxter. -

A Selection of New Arrivals September 2017
A selection of new arrivals September 2017 Rare and important books & manuscripts in science and medicine, by Christian Westergaard. Flæsketorvet 68 – 1711 København V – Denmark Cell: (+45)27628014 www.sophiararebooks.com AMPERE, Andre-Marie. Mémoire. INSCRIBED BY AMPÈRE TO FARADAY AMPÈRE, André-Marie. Mémoire sur l’action mutuelle d’un conducteur voltaïque et d’un aimant. Offprint from Nouveaux Mémoires de l’Académie royale des sciences et belles-lettres de Bruxelles, tome IV, 1827. Bound with 18 other pamphlets (listed below). [Colophon:] Brussels: Hayez, Imprimeur de l’Académie Royale, 1827. $38,000 4to (265 x 205 mm). Contemporary quarter-cloth and plain boards (very worn and broken, with most of the spine missing), entirely unrestored. Preserved in a custom cloth box. First edition of the very rare offprint, with the most desirable imaginable provenance: this copy is inscribed by Ampère to Michael Faraday. It thus links the two great founders of electromagnetism, following its discovery by Hans Christian Oersted (1777-1851) in April 1820. The discovery by Ampère (1775-1836), late in the same year, of the force acting between current-carrying conductors was followed a year later by Faraday’s (1791-1867) first great discovery, that of electromagnetic rotation, the first conversion of electrical into mechanical energy. This development was a challenge to Ampère’s mathematically formulated explanation of electromagnetism as a manifestation of currents of electrical fluids surrounding ‘electrodynamic’ molecules; indeed, Faraday directly criticised Ampère’s theory, preferring his own explanation in terms of ‘lines of force’ (which had to wait for James Clerk Maxwell (1831-79) for a precise mathematical formulation). -

The Last Beethoven Overture This Study Is, Above All, the Outcome of A
1 The Last Beethoven Beethoven, deaf, working on the manuscript of the Missa Solemnis (painting by Josef Karl Stieler, 1819) Overture This study is, above all, the outcome of a long-lasting personal concern that goes back to the period of my first music lessons and my attempts to play Beethoven’s music on the piano. My passion for certain musical compositions, the care to interpret them in the way, with the sensitivity and in keeping with their creator’s intentions might be translated as follows: I wish to perform as if he could hear me and could recognize himself in the music I performed. I was filled, at a very early age, with the desire to know as much as possible about his personality, his life, the events and the circumstances that led to the birth of his work. It is obvious and easy to prove, based on Beethoven’s own notes and the testimonies of those who have written about him for nearly two hundred years, that there were external triggers, such as social and even historical events or happenings, which activated certain musical themes that his genius and sensitivity gave expression in the form known to us today. In this study, I will speak, at the appropriate time, about situations, contexts and events of this kind: family problems, like the affair involving his nephew Karl, 2 or sentimental issues, like the “Immortal Beloved” (Der Unsterbliche Geliebte), the drama entailed by hearing loss, the evolution of event on the European stage during the Napoleonic and post-Napoleonic periods, etc. -

Albert Einstein - Wikipedia, the Free Encyclopedia Page 1 of 27
Albert Einstein - Wikipedia, the free encyclopedia Page 1 of 27 Albert Einstein From Wikipedia, the free encyclopedia Albert Einstein ( /ælbərt a nsta n/; Albert Einstein German: [albt a nʃta n] ( listen); 14 March 1879 – 18 April 1955) was a German-born theoretical physicist who developed the theory of general relativity, effecting a revolution in physics. For this achievement, Einstein is often regarded as the father of modern physics.[2] He received the 1921 Nobel Prize in Physics "for his services to theoretical physics, and especially for his discovery of the law of the photoelectric effect". [3] The latter was pivotal in establishing quantum theory within physics. Near the beginning of his career, Einstein thought that Newtonian mechanics was no longer enough to reconcile the laws of classical mechanics with the laws of the electromagnetic field. This led to the development of his special theory of relativity. He Albert Einstein in 1921 realized, however, that the principle of relativity could also be extended to gravitational fields, and with his Born 14 March 1879 subsequent theory of gravitation in 1916, he published Ulm, Kingdom of Württemberg, a paper on the general theory of relativity. He German Empire continued to deal with problems of statistical Died mechanics and quantum theory, which led to his 18 April 1955 (aged 76) explanations of particle theory and the motion of Princeton, New Jersey, United States molecules. He also investigated the thermal properties Residence Germany, Italy, Switzerland, United of light which laid the foundation of the photon theory States of light. In 1917, Einstein applied the general theory of relativity to model the structure of the universe as a Ethnicity Jewish [4] whole. -

Towards a Co-Creative Encounter with the Book by Cameron Martin
Gone Critical— Towards a Co-Creative Encounter with the Book by Cameron Martin Reid A thesis presented to the University of Waterloo in fulfilment of the thesis requirement for the degree of Doctor of Philosophy in English Waterloo, Ontario, Canada, 2010 ©Cameron Martin Reid 2010 I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by my examiners. I understand that my thesis may be made electronically available to the public. ii Abstract This dissertation follows two interrelated lines of inquiry. The first, I formulate as follows: (1) How, historically speaking, has the discourse of literary criticism thought the book? How has it represented the book? Used the book? Put simply, what has the book become in the hands of the critic? Though, of course, answers to such questions will vary widely—especially as they intersect with related matters concerning the critic, herself, and what Henry Sussman refers to as the perceived ―task of the critic‖—it is my contention that the discourse of literary criticism remains unified by its inability to extricate itself from what I call the transcendent orientation to literature: an orientation that has both ancient and modern coordinates. In Part 1 of the dissertation, I map criticism‘s ongoing historical affair with transcendence—an affair that begins as far back as the Platonic dialogues, but that can be traced right up through the twentieth century, in and through the work of any number of critics, and many prominent schools of literary critical thought. -
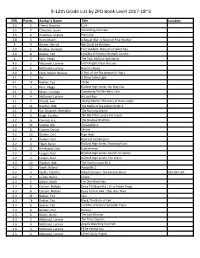
9-12Th Grade List by ZPD Book Level 2017-18~3
9-12th Grade List by ZPD Book Level 2017-18~3 ZPD Points Author's Name Title Location 3.2 5 Friend, Natasha Lush 3.5 7 Colasanti, Susan Something Like Fate 3.5 6 Hamilton, Virginia Plain City 3.8 3 Harry Mazer A Boy at War: A Novel of Pearl Harbor 4 4 Barnes, Derrick We Could be Brothers 4.0 5 Bradley, Kimberly For Freedom: Story of a French Spy 4.0 8 Dekker, Ted Lost Bks of History Series#5: Lunatic 4 3 Kern, Peggy The Test, Bluford High Series 4.0 5 McDaniel, Lurlene Until Angels Close my Eyes 4.0 5 McDaniel, Lurlene Heart to Heart 4.0 4 Peck, Robert Newton A Part of the Sky (sequel to Pigs ) 4.1 5 Avi A Place Called Ugly 4.1 14 Dekker, Ted Thr3e 4.1 3 Kern, Peggy Bluford High Series: No Way Out 4.1 4 Mazer, Lerangis Somebody Tell Me Who I Am 4.1 4 McDaniel, Lurlene Hit and Run 4.1 7 Rinaldi, Ann Taking Liberty: The Story of Oney Judge 4.1 12 Riordan, Rick The Battle of the Labyrinth Bk 4 4.1 9 Van Draanen, Wendelin The Running Dream 4.1 9 Voigt, Cynthia Tell Me if the Lovers are Losers 4.2 6 Cannon, A.E. The Shadow Brothers 4.2 13 Condie, Ally Crossed Bk 2 4.2 8 Cooner, Donna Skinny 4.2 10 Deuker, Carl High Heat 4.2 8 Deuker, Carl Heart of a Champion 4.2 4 Folan, Karyn Bluford High Series: Breaking Point 4.2 12 Honeyman, Kay Interference 4.2 3 Langan, Paul Bluford High Series: Search for Safety 4.2 3 Langan, Paul Bluford High Series: The Fallen 4.2 10 Riordan, Rick The Titan’s Curse Bk 3 4.2 10 Smith, Roland Above Bk 2 4.2 5 Yeatts, Tabatha Albert Einstein: The Miracle Mind 530.092 YEA 4.2 6 Lo'pez, Diana Choke 4.3 5 Albom, Mitch For