Soil Respiration in an Old-Growth Subtropical Forest
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

XUE Liang Ph.D., Associate Professor
XUE Liang Ph.D., Associate Professor Email: [email protected] Address of Office: Room 404 in the Zhongyou Building, Department of Petroleum Engineering,18 Fuxue Road, Changping District, Beijing 102249,China Education Ph.D., Hydrology, University of Arizona (United States), 2007 M.S., Environmental Engineering, China University of Geosciences - Beijing (China), 2005 B.S., Environmental Engineering, China University of Geosciences - Beijing (China), 2001 Research Areas and Interests Subsurface flow and transport in porous and fractured media Automatic history matching Machine Learning Stochastic analysis and optimization Teaching Fluid mechanics in porous media Professional English for petroleum engineering Academic writing for petroleum engineering Professional Experiences 2007-2011, University of Arizona, Research Assistant 2012-2014, College of Engineering, Peking University, Postdoc 2014-2015, Assistant Professor, Department of Petroleum Engineering, China University of Petroleum-Beijing, China 2015-present, Associate Professor, Department of Petroleum Engineering, China University of Petroleum-Beijing, China Other Professional Affiliations Member of American Geophysical Union Member of Society of Petroleum Engineering Selected Publications 1. Cheng Dai,Liang Xue,Weihong Wang,Xiang Li. Analysis of the influencing factors on the well performance in shale gas reservoir. Geofluids,2016.12,0(0):1~12 2. Liang Xue,Diao Li,Cheng Dai,Tongchao Nan,Characterization of Aquifer Multiscale Properties by Generating Random Fractal Field with Truncated Power Variogram Model Using Karhunen–Loève Expansion,Geofluids,2017.12, 0(0):1~15 3. Liang Xue,Cheng Dai,Lei Wang,Development of a General Package for Resolution of Uncertainty-Related Issues in Reservoir Engineering,Energies,2017.2.10,10(2):197~212 4. Xuan Liu,Cheng Dai,Liang Xue,Bingyu Ji,Estimation of fracture distribution in a CO2‐EOR system through Ensemble Kalman filter,Greenhouse Gases Science & Technology,2017.10.10,0:1-22 5. -

Liang Kevin Guo
LIANG KEVIN GUO CONTACT INFORMATION Department of Accounting and Finance Cell Phone: (617) 820-2622 Jack H. Brown College Office Phone: (909) 537-3257 Business & Public Administration Fax: (210) 458-6320 California State University, San Bernardino 5500 E-mail: [email protected] University Parkway, San Bernardino, CA Office Location: JB 225 EDUCATION The University of Texas at San Antonio, College of Business San Antonio, TX Ph.D. in Finance, August 2013 2009 - 2013 Charter Financial Analyst (CFA) Boston University, School of Management Boston, MA M.B.A., Concentration in Finance, May 2009 2007 - 2009 Graduation with High Honor Peking University, School of Economics Beijing, China B.S. in Economics, July 2002 1998 - 2002 TEACHING EXPERIENCE Associate Finance Professor, Jack. H. Brown College of Business and Public Administration (JHBC), California State University, San Bernardino (CSUSB), CA Fall 2017 – Present Assistant Finance Professor, Jack. H. Brown College of Business and Public Administration (JHBC), California State University, San Bernardino (CSUSB), CA Fall 2013 – Spring 2017 FIN 313: Business Finance (Teaching Evaluation: 5.8 / 6.0) FIN 314: Corporate Finance Management (Teaching Evaluation: 5.8 / 6.0) FIN 430: Financial Theory and Practice (Teaching Evaluation: 6.0 / 6.0) FIN 435: Investment Analysis (Teaching Evaluation: 5.6 / 6.0) FIN 546 Student Investment Fund Instructor, University of Texas at San Antonio, TX Summer 2012 –Summer 2013 FIN 3033: Principles of Investment (Teaching Evaluation: 4.59 / 5.0) FIN 3023: Intermediate -

The Later Han Empire (25-220CE) & Its Northwestern Frontier
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2012 Dynamics of Disintegration: The Later Han Empire (25-220CE) & Its Northwestern Frontier Wai Kit Wicky Tse University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Asian History Commons, Asian Studies Commons, and the Military History Commons Recommended Citation Tse, Wai Kit Wicky, "Dynamics of Disintegration: The Later Han Empire (25-220CE) & Its Northwestern Frontier" (2012). Publicly Accessible Penn Dissertations. 589. https://repository.upenn.edu/edissertations/589 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/589 For more information, please contact [email protected]. Dynamics of Disintegration: The Later Han Empire (25-220CE) & Its Northwestern Frontier Abstract As a frontier region of the Qin-Han (221BCE-220CE) empire, the northwest was a new territory to the Chinese realm. Until the Later Han (25-220CE) times, some portions of the northwestern region had only been part of imperial soil for one hundred years. Its coalescence into the Chinese empire was a product of long-term expansion and conquest, which arguably defined the egionr 's military nature. Furthermore, in the harsh natural environment of the region, only tough people could survive, and unsurprisingly, the region fostered vigorous warriors. Mixed culture and multi-ethnicity featured prominently in this highly militarized frontier society, which contrasted sharply with the imperial center that promoted unified cultural values and stood in the way of a greater degree of transregional integration. As this project shows, it was the northwesterners who went through a process of political peripheralization during the Later Han times played a harbinger role of the disintegration of the empire and eventually led to the breakdown of the early imperial system in Chinese history. -

English Versions of Chinese Authors' Names in Biomedical Journals
Dialogue English Versions of Chinese Authors’ Names in Biomedical Journals: Observations and Recommendations The English language is widely used inter- In English transliteration, two-syllable Forms of Chinese Authors’ Names nationally for academic purposes. Most of given names sometimes are spelled as two in Biomedical Journals the world’s leading life-science journals are words (Jian Hua), sometimes as one word We recently reviewed forms of Chinese published in English. A growing number (Jianhua), and sometimes hyphenated authors’ names accompanying English- of Chinese biomedical journals publish (Jian-Hua). language articles or abstracts in various abstracts or full papers in this language. Occasionally Chinese surnames are Chinese and Western biomedical journals. We have studied how Chinese authors’ two syllables (for example, Ou-Yang, Mu- We found considerable inconsistency even names are presented in English in bio- Rong, Si-Ma, and Si-Tu). Editors who are within the same journal or issue. The forms medical journals. There is considerable relatively unfamiliar with Chinese names were in the following categories: inconsistency. This inconsistency causes may mistake these compound surnames for • Surname in all capital letters followed by confusion, for example, in distinguishing given names. hyphenated or closed-up given name, for surnames from given names and thus cit- China has 56 ethnic groups. Names example, ing names properly in reference lists. of minority group members can differ KE Zhi-Yong (Chinese Journal of In the current article we begin by pre- considerably from those of Hans, who Contemporary Pediatrics) senting as background some features of constitute most of the Chinese population. GUO Liang-Qian (Chinese Chinese names. -
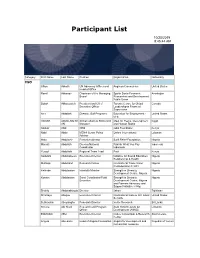
Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -

Chih-Liang Liu
CHIH-LIANG LIU Institute for Financial & Accounting Studies Tel: (+86)592-2187352 Xiamen University E-mail: [email protected] Room 511-4, Jiageng Building 2, 422 Siming South Road, Xiamen, Fujian, 361005 China EDUCATION Ph.D., Accounting, National Taiwan University, Taipei, Taiwan, 2007 ACADEMIC POSITION Assistant Professor in Accounting, Institute for Financial & Accounting Studies, Xiamen University, China TEACHING COURSES Undergraduate level: Financial Accounting Postgraduate level: Financial Statement Analysis PROFESSIONAL AFFILIATIONS Member, American Accounting Association, U.S.A. (2008-present) RESEARCH INTERESTS Financial Accounting: Accounting conservatism, auditor quality, board connection, corporate governance, internal controls, investment efficiency, managerial characteristics PUBLISHED RESEARCH ARTICLES Shu-Miao Lai and Chih-Liang Liu*. 2018. The Effect of Auditor Characteristics on the Value of Diversification. A Auditing: A Journal of Practice and Theory 37 (1): 115-137. (A* journal indexed in Scopus and SSCI) (*Corresponding author) Shu-Miao Lai and Chih-Liang Liu*. 2018. Management Characteristics and Corporate Investment Efficiency. Asia-Pacific Journal of Accounting and Economics 25 (3-4): 295–312 (Lead article). (SSCI) (*Corresponding author) Yi-Mien Lin, Chih-Chen Lee, Chin-Fang Chao and Chih-Liang Liu. 2015. The Information Content of Unexpected Stock Returns: Evidence from Intellectual Capital. International Review of Economics and Finance 37: 208–225. (SSCI) Shu-Miao Lai, Chih-Liang Liu* and Taychang Wang. 2014. Increased Disclosure and Investment Efficiency. Asia-Pacific Journal of Accounting and Economics 21 (3): 308–327. (SSCI) (*Corresponding author) Yi-Mien Lin, Chin-Fang Chao and Chih-Liang Liu. 2014. Transparency, Idiosyncratic Risk, and Convertible Bonds. The European Journal of Finance 20 (1): 80–103. -

Detecting Digital Fingerprints: Tracing Chinese Disinformation in Taiwan
Detecting Digital Fingerprints: Tracing Chinese Disinformation in Taiwan By: A Joint Report from: Nick Monaco Institute for the Future’s Digital Intelligence Lab Melanie Smith Graphika Amy Studdart The International Republican Institute 08 / 2020 Acknowledgments The authors and organizations who produced this report are deeply grateful to our partners in Taiwan, who generously provided time and insights to help this project come to fruition. This report was only possible due to the incredible dedication of the civil society and academic community in Taiwan, which should inspire any democracy looking to protect itself from malign actors. Members of this community For their assistance in several include but are not limited to: aspects of this report the authors also thank: All Interview Subjects g0v.tw Projects Gary Schmitt 0archive Marina Gorbis Cofacts Nate Teblunthuis DoubleThink Lab Sylvie Liaw Taiwan FactCheck Center Sam Woolley The Reporter Katie Joseff Taiwan Foundation for Democracy Camille François Global Taiwan Institute Daniel Twining National Chengchi University Election Johanna Kao Study Center David Shullman Prospect Foundation Adam King Chris Olsen Hsieh Yauling The Dragon’s Digital Fingerprint: Tracing Chinese Disinformation in Taiwan 2 Graphika is the network Institute for the Future’s The International Republican analysis firm that empowers (IFTF) Digital Intelligence Lab Institute (IRI) is one of the Fortune 500 companies, (DigIntel) is a social scientific world’s leading international Silicon Valley, human rights research entity conducting democracy development organizations, and universities work on the most pressing organizations. The nonpartisan, to navigate the cybersocial issues at the intersection of nongovernmental institute terrain. With rigorous and technology and society. -

Intellectual Property Rights, Complementarity and the Firm's
136 Int. J. Intellectual Property Management, Vol. 9, No. 2, 2019 Intellectual property rights, complementarity and the firm’s economic performance Liang Guo-Fitoussi, Ahmed Bounfour* and Sabrine Rekik Faculté Jean Monnet, Université Paris-Sud, Universityé Paris-Saclay, 54 Bd Desgranges, 92330, Sceaux, France Email: [email protected] Email: [email protected] Email: [email protected] *Corresponding author Abstract: This paper analyses the optimal use of formal intellectual property rights (IPR) at the firm level. We examine the impact of combinations of IPR on the firm’s productivity in order to study the complementarity or substitution relationship between them. Our data are extracted from two sets of community innovation survey (CIS): CIS IV and CIS 2006. We investigate complementarity (substitution) at two levels: in the context of formal IPR strategies and when they are combined with other intangible and managerial assets based on both adoption and productivity approaches. Mixed results are found for the complementarity of IPR alone. However, in combination with other activities, there is high complementarity with innovative and innovation cooperation variables. These results suggest that IPR strategies should be combined with other complementarity assets for the optimal appropriation of innovation profits. Keywords: intellectual property rights; IPR; innovation; complementarity; substitution; economic performance; intangible assets. Reference to this paper should be made as follows: Guo-Fitoussi, L., Bounfour, A. and Rekik, S. (2019) ‘Intellectual property rights, complementarity and the firm’s economic performance’, Int. J. Intellectual Property Management, Vol. 9, No. 2, pp.136–165. Biographical notes: Liang Guo-Fitoussi holds a PhD in Economics of Université Paris Ouest-La Défense. -

The Saxophone in China: Historical Performance and Development
THE SAXOPHONE IN CHINA: HISTORICAL PERFORMANCE AND DEVELOPMENT Jason Pockrus Dissertation Prepared for the Degree of DOCTOR OF MUSICAL ARTS UNIVERSITY OF NORTH TEXAS August 201 8 APPROVED: Eric M. Nestler, Major Professor Catherine Ragland, Committee Member John C. Scott, Committee Member John Holt, Chair of the Division of Instrumental Studies Benjamin Brand, Director of Graduate Studies in the College of Music John W. Richmond, Dean of the College of Music Victor Prybutok, Dean of the Toulouse Graduate School Pockrus, Jason. The Saxophone in China: Historical Performance and Development. Doctor of Musical Arts (Performance), August 2018, 222 pp., 12 figures, 1 appendix, bibliography, 419 titles. The purpose of this document is to chronicle and describe the historical developments of saxophone performance in mainland China. Arguing against other published research, this document presents proof of the uninterrupted, large-scale use of the saxophone from its first introduction into Shanghai’s nineteenth century amateur musical societies, continuously through to present day. In order to better describe the performance scene for saxophonists in China, each chapter presents historical and political context. Also described in this document is the changing importance of the saxophone in China’s musical development and musical culture since its introduction in the nineteenth century. The nature of the saxophone as a symbol of modernity, western ideologies, political duality, progress, and freedom and the effects of those realities in the lives of musicians and audiences in China are briefly discussed in each chapter. These topics are included to contribute to a better, more thorough understanding of the performance history of saxophonists, both native and foreign, in China. -

Names of Chinese People in Singapore
101 Lodz Papers in Pragmatics 7.1 (2011): 101-133 DOI: 10.2478/v10016-011-0005-6 Lee Cher Leng Department of Chinese Studies, National University of Singapore ETHNOGRAPHY OF SINGAPORE CHINESE NAMES: RACE, RELIGION, AND REPRESENTATION Abstract Singapore Chinese is part of the Chinese Diaspora.This research shows how Singapore Chinese names reflect the Chinese naming tradition of surnames and generation names, as well as Straits Chinese influence. The names also reflect the beliefs and religion of Singapore Chinese. More significantly, a change of identity and representation is reflected in the names of earlier settlers and Singapore Chinese today. This paper aims to show the general naming traditions of Chinese in Singapore as well as a change in ideology and trends due to globalization. Keywords Singapore, Chinese, names, identity, beliefs, globalization. 1. Introduction When parents choose a name for a child, the name necessarily reflects their thoughts and aspirations with regards to the child. These thoughts and aspirations are shaped by the historical, social, cultural or spiritual setting of the time and place they are living in whether or not they are aware of them. Thus, the study of names is an important window through which one could view how these parents prefer their children to be perceived by society at large, according to the identities, roles, values, hierarchies or expectations constructed within a social space. Goodenough explains this culturally driven context of names and naming practices: Department of Chinese Studies, National University of Singapore The Shaw Foundation Building, Block AS7, Level 5 5 Arts Link, Singapore 117570 e-mail: [email protected] 102 Lee Cher Leng Ethnography of Singapore Chinese Names: Race, Religion, and Representation Different naming and address customs necessarily select different things about the self for communication and consequent emphasis. -

Rethinking Chinese Kinship in the Han and the Six Dynasties: a Preliminary Observation
part 1 volume xxiii • academia sinica • taiwan • 2010 INSTITUTE OF HISTORY AND PHILOLOGY third series asia major • third series • volume xxiii • part 1 • 2010 rethinking chinese kinship hou xudong 侯旭東 translated and edited by howard l. goodman Rethinking Chinese Kinship in the Han and the Six Dynasties: A Preliminary Observation n the eyes of most sinologists and Chinese scholars generally, even I most everyday Chinese, the dominant social organization during imperial China was patrilineal descent groups (often called PDG; and in Chinese usually “zongzu 宗族”),1 whatever the regional differences between south and north China. Particularly after the systematization of Maurice Freedman in the 1950s and 1960s, this view, as a stereo- type concerning China, has greatly affected the West’s understanding of the Chinese past. Meanwhile, most Chinese also wear the same PDG- focused glasses, even if the background from which they arrive at this view differs from the West’s. Recently like Patricia B. Ebrey, P. Steven Sangren, and James L. Watson have tried to challenge the prevailing idea from diverse perspectives.2 Some have proven that PDG proper did not appear until the Song era (in other words, about the eleventh century). Although they have confirmed that PDG was a somewhat later institution, the actual underlying view remains the same as before. Ebrey and Watson, for example, indicate: “Many basic kinship prin- ciples and practices continued with only minor changes from the Han through the Ch’ing dynasties.”3 In other words, they assume a certain continuity of paternally linked descent before and after the Song, and insist that the Chinese possessed such a tradition at least from the Han 1 This article will use both “PDG” and “zongzu” rather than try to formalize one term or one English translation. -

Proquest Dissertations
INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with small overlaps. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6” x 9” black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. Bell & Howell Information and Learning 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 800-521-0600 UMI" ARGUMENT STRUCTURE, HPSG, AND CHINESE GRAMMAR DISSERTATION Presented in Partial Fulfilment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University by Qian Gao, B.A., M.A. ******* The Ohio State University 2001 Dissertation Committee: Approved by Professor Carl J. Pollard, Adviser Professor Peter W.