Chemistry 2B Lab Manual Standard Operating Procedures Winter Quarter 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protocol for Use of Differential Scanning Calorimeter (NSF/Epscor Proteomics Facility @ Brown University)
MicroCal VP-DSC Geoff Stetson/ Page Laboratory/ 2007 Protocol for Use of Differential Scanning Calorimeter (NSF/EPSCoR Proteomics Facility @ Brown University) 1. Equipment (photos) 2. Getting Started Degasser - Turn on the degasser. - Make sure the metal valve on the top of the lid is closed. - Set the temperature to 20-25°C. - Place a small stir bar in a couple of plastic vials. - Fill vials 2/3 full with Milli-Q water. - Place vials into slots on top of degasser. - Turn the stirrer speed knob to - 10 or 11 o’clock. - - Place the lid on firmly, and while pressing down turn on the degasser o (Note: If you flip the switch to on, the degasser will run until switched off. If the switch is flipped to timer, the degasser will run for eight minutes). - Degas the sample for 8-15 minutes. o Turn off the vacuum. - Open the metal valve on top of the lid in order to release the vacuum created underneath. - Remove the plastic vials. 1 MicroCal VP-DSC Geoff Stetson/ Page Laboratory/ 2007 DSC - Unscrew top. - Remove contents of of the sample cell and the reference cell using the glass filling syringe. o Insert the funnel into the top of the cell. o Slowly put the syringe into the cell until it gently touches the bar across the top of the funnel, then remove the liquid. Repeat. o (Note: Be careful when putting anything into the cells. The machine is extremely sensitive.) - Fill glass syringe with degassed Milli-Q water. - Rinse out both cells with the Milli-Q water (3x). -

Soda Can Calorimeter
Soda Can Calorimeter Energy Content of Food SCIENTIFIC SCIENCEFAX! Introduction Have you ever noticed the nutrition label located on the packaging of the food you buy? One of the first things listed on the label are the calories per serving. How is the calorie content of food determined? This activity will introduce the concept of calorimetry and investigate the caloric content of snack foods. Concepts •Calorimetry • Conservation of energy • First law of thermodynamics Background The law of conservation of energy states that energy cannot be created or destroyed, only converted from one form to another. This fundamental law was used by scientists to derive new laws in the field of thermodynamics—the study of heat energy, temperature, and heat transfer. The First Law of Thermodynamics states that the heat energy lost by one body is gained by another body. Heat is the energy that is transferred between objects when there is a difference in temperature. Objects contain heat as a result of the small, rapid motion (vibrations, rotational motion, electron spin, etc.) that all atoms experience. The temperature of an object is an indirect measurement of its heat. Particles in a hot object exhibit more rapid motion than particles in a colder object. When a hot and cold object are placed in contact with one another, the faster moving particles in the hot object will begin to bump into the slower moving particles in the colder object making them move faster (vice versa, the faster particles will then move slower). Eventually, the two objects will reach the same equilibrium temperature—the initially cold object will now be warmer, and the initially hot object will now be cooler. -

T E M P E R a T U
THE HIGH TEMPERATURE HEAT CONTENTS OP 0 MOLYBDENUM AND TITANIUM AND THE LOW TEMPERATURE HEAT CAPACITIES OP TITANIUM DISSERTATION Presented In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By CHARLES WILLIAM KOTHEN, B.A. // The Ohio State University 1952 Approved By: Adviser i TABLE OF CONTENTS E&gft INTRODUCTION ............................ 1 THEORETICAL ............................. 3 HISTORICAL .............................. 6 PART I The High Temperature Heat Contents of Molybdenum and Titahium ................... 13 Introduction ...................... 13 Apparatus .......................... 14 Measurements and Calculations ........ 32 Errors ... ......................... 45 Experimental Results ............... 49 PART II Low Temperature Heat Capacity of Titanium.. 61 Introduction ...................... 61 Apparatus ........................... 62 Measurements and Calculations ....... 71 Errors ................... 74 Experimental Results ............... 75 ACKNOWLEDGMENTS .......................... 80 APPENDIX I Physical Constants, Drop Calorimeter Data .... 61 APPENDIX II Low Temperature Calorimeter Data ..... 82 APPENDIX III Standard Lamp Calibration ............ 83 3182G G TABLE OF CONTENTS, (cont.) Page APPENDIX IV Bibliography ................. 85 AUTOBIOGRAPHY .......................... 89 iii LIST OF ILLUSTRATIONS 1 Vacuum Furnace 15 2 Dropping Mechanism 19 3 Improved Calorimeter 20 4. Modified Calorimeter, II 21 5 Drop Calorimeter Electrical Circuits -

LAB: One Tube Reaction Part 1
AP Chemistry LAB: One Tube Reaction Part 1 Objective: To monitor and document the chemical changes occurring in a single test tube containing a predetermined mixture of chemicals. Materials: test tube, iron nail, sodium chloride, copper (II) sulfate, distilled water, glass stirring rod, tissue paper, parafilm wax Procedures: 1. Label a test tube to identify your group 2. Fill approx. 1/3 of the test tube with copper (II) sulfate crystals. Gently tap the tube to allow the crystals to settle. 3. Using a glass stirring rod, carefully cover the crystals with a layer of Kimwipe tissue paper. 4. Slowly, and with as little disturbance as possible, add enough distilled water to just cover the paper and blue crystals. 5. Repeat the process for the sodium chloride, filling approx. 1/3 of the test tube. Gently tap the tube to allow the crystals to settle. 6. Push more tissue paper into the test tube on top of the white crystals. 7. Add enough water to cover the tissue paper and white crystals. 8. Obtain an iron nail and expose the surface by rubbing with sand paper. 9. Carefully slide the nail into the test tube. 10. Continue adding water until the nail is completely covered. 11. Cover the test tube with parafilm wax and record your Day 1 observations. 12. Return the test tube to the test tube rack. 13. Continue to monitor and record your observations for the next several days, as indicated in the data table. Data: 1. Complete the NFPA label for the two chemicals used in this lab. -

Adiabatic Dewar Calorimeter
I.CHEM.E. SYMPOSIUM SERIES NO. 97 ADIABATIC DEWAR CALORIMETER T.K.Wright'and R.L.Rogers* A simple calorimeter has been developed that enables chemical reaction runaway conditions to be directly determined, under the low heat loss conditions found in full scale chemical plants. Since the calorimeter provides temperature time data in the near absence of environmental heat losses the data can be simply analysed to yield heats of reaction and chemical power output. The latter are used either in conjunction with plant natural cooling data to assess reactor stability or at higher temperatures to size reactor vents. If the reaction mechanisms are known or adequate assumptions can be made then the temperature-time data can also be processed to yield reaction kinetics constants for simulation purposes. Keywords: Hazards, Exotherms, Adiabatic Calorimeter, kinetics INTRODUCTION Evaluation of chemical reaction hazards requires the detection of exotherms/gas generation likely to lead to reactor overpressure. Some form of small scale scanning calorimetry is generally used for the initial detection of the exotherm and gas generation and a temperature of onset will be determined which is dependent on the sensitivity of the equipment, but on a 10-20gm scale exothermlcity will generally be detected at self heating rates of 2-10°C/hr - approximately 3-10 watts/lit. Depending on apparent exotherm size and proximity to process temperature or any likely excursions then secondary testing may be required to display more accurately (a) The minimum temperature above which the reactor will be unstable on the scale used. (b) The consequences of the exotherm - heat of reaction/adiabatic rise/ pressure developed/venting requirement. -

Equipment Detailsr07
Lab Equipment Details Lab Equipment Glass Flasks 150ml 250ml 500ml Lab Equipment Glass Beakers 150ml 250ml 500ml Lab Equipment Glassware But once removed, only the cap stays highlighted. Droppers critical to Lab An activated Dropper coursework can be found highlights the entire bottle. already in the workspace. Dropper Dropper Dropper Activated In Use Lab Equipment Gastight Syringe Small A pre-filled gastight syringe can be used with a NMR tube to safely fill through the top in preparation for use with the NMR spectrometer. Gastight Syringe NMR Tube with Holder with Holder Lab Equipment NMR Tube Spinner An NMR Tube filled with gas for use with the NMR Spectrometer Simply use the NMR Tube with needs to be inserted into the the Tube Spinner. Spinner before it can be used. NMR Tube NMR Tube Spinner NMR Tube Spinner with Holder with NMR Tube inserted Once the Tube slotted into the holder is inserted into the top part of the spectrometer, users can type in a number for Lab Equipment the frequency they’d like to scan. NMR Spectrometer XL The NMR Tube holder can then be slotted into the highlighted tube on the NMR Spectrometer. NMR Tube filled with appropriate substance is slotted (used with) in the holder. Lift will perpare the Spectrometer for the tube holder insertion. Scan No. Allows the user to change the frequency at which the tube is scanned The NMR Tube holder can then be slotted into the top tube on Lab Equipment the NMR Spectrometer. NMR Spectrometer XL NMR Tube filled with appropriate substance is slotted (used with) in the holder. -

Linseis Optical Dilatometer and Heating Microscope Brochure
THERMAL ANALYSIS Optical Dilatometer DIL L74 Heating Microscope Since 1957 LINSEIS Corporation has been deliv- ering outstanding service, know how and lead- ing innovative products in the field of thermal analysis and thermo physical properties. Customer satisfaction, innovation, flexibility and high quality are what LINSEIS represents. Thanks to these fundamentals our company enjoys an exceptional reputation among the leading scientific and industrial organizations. LINSEIS has been offering highly innovative benchmark products for many years. The LINSEIS business unit of thermal analysis is involved in the complete range of thermo Claus Linseis analytical equipment for R&D as well as qual- Managing Director ity control. We support applications in sectors such as polymers, chemical industry, inorganic building materials and environmental analytics. In addition, thermo physical properties of solids, liquids and melts can be analyzed. LINSEIS provides technological leadership. We develop and manufacture thermo analytic and thermo physical testing equipment to the high- est standards and precision. Due to our innova- tive drive and precision, we are a leading manu- facturer of thermal Analysis equipment. The development of thermo analytical testing machines requires significant research and a high degree of precision. LINSEIS Corp. invests in this research to the benefit of our customers. 2 German engineering Innovation The strive for the best due diligence and ac- We want to deliver the latest and best tech- countability is part of our DNA. Our history is af- nology for our customers. LINSEIS continues fected by German engineering and strict quality to innovate and enhance our existing thermal control. analyzers. Our goal is constantly develop new technologies to enable continued discovery in Science. -

Simple Calorimeter for Heats of Fusion. Data on The
U.S. Department of Commerce, Bureau of Standards RESEARCH PAPER RP607 Part of Bureau of Standards Journal of Research, Vol. 11, October 1933 A SIMPLE CALORIMETER FOR HEATS OF FUSION. DATA ON THE FUSION OF PSEUDOCUMENE, MESITYLENE (« AND 0), HEMIMELLITENE, o- AND m-XYLENE, AND ON TWO TRANSITIONS OF HEMIMELLITENE By Frederick D. Rossini abstract A vacuum flask with a thermoelement serves as a simple calorimeter for measuring heats of fusion quickly and economically, with an accuracy of a few percent. The following heats of fusion (with estimated uncertainties), in k-cal. per mole, were obtained: pseudocumene, — 44.1° C, 2.75±0.06; hemimelli- tene,-25.5° C, 2.00±0.05; mesitylene (a), — 44.8° C, 2.28±0.06; mesitylene (0), -51.7° C., 1.91±0.05; o-xylene,-25.3° C, 3.33±0.07; m-xylene,-47.9° C, 2.76 ±0.05. Hemimellitene was found to have two transitions below the freez- ing point, with the following heats of transition, in A>cal. per mole: hemimelli- tene (7-»0),-58±2° C.,0.28±0.04; hemimellitene (P^a),- 46 ± 1° C.,0.36±0.04. CONTENTS Page I. Introduction 553 II. Apparatus and method 553 III. Materials 554 IV. Standardization experiments 555 V. Experimental data 557 VI. Conclusion 559 I. INTRODUCTION The simple calorimeter described here was assembled in order to provide a means for measuring as quickly and economically as prac- ticable, and with an accuracy of a few percent, the heats of fusion of certain hydrocarbons for which there are no data. -

The Measurement of Heat Release Rates by Oxygen Consumption Calorimetry in Fires Under Suppression
The Measurement of Heat Release Rates by Oxygen Consumption Calorimetry in Fires Under Suppression BOGDAN Z. DLUGOGORSKI, JACK R. MAWHINNEY and VO HUU DUC National Research Counc~l,Institute for Research in Construction Ottawa, Ontario KIA OR6, Canada ABSTRACT A series of open-space fire experiments was conducted at the National Fire Laboratory (NFL) to validate the capabilities of the NFL room-size oxygen-consumption calorimeter, and to assess the importance of accounting for actual water vapour content in the exhaust gases in calculating heat release rates (HRR). Water spray was used to partially suppress some of the fires, and to add significantly to the humidity of the exhaust gases. The equations normally used in the fire research community for oxygen calorimeter assume unsuppressed fires, and that water vapour in the exhaust gases is due solely to the humidity of the incoming air and to combustion reactions. This paper derives the basic equations for computing heat release rates based on the principle of nitrogen balance. The general equations take into account all sources of water vapour, including incoming air, combustion reactions, and evaporation due to suppression. The equations are then simplified to i) neglect all humidity, and, ii) consider only the humidity of the incoming air. The predictions of the HRR from the three sets of equations are compared with the HRR calculated for unsuppressed fires and with the HRR obtained by measuring fuel consumption rates. As long as the water vapour content in the exhaust gases is less than 7 %, both simplified equations can be used to measure the HRR of partially suppressed fires, without significant error. -

Documm Mgson!
Documm mgson! ED 174 412 SE 027 981 TITLE Biomedical Science, Unit I: Respiration inHealth and Medicine. Respiratory Anatomy, Physiology and Pathology; The Behavior of Gases;Introductory Chemistry; and Air Pollution. Laboratory Manual. Revised Version, 1975. INSTITUTION Biomedical Interdisciplinary Curriculum Project, Berkeley, Calif. SPONS AGENCY National Science Foundation, Washington, D.C. PUB DATE 75 NOTE 181p.; For related documents, see SE 027978-999 and SE 028 510-516; Not available in hard copydue to copyright restrictions EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIpTCRS Chemistry; Environment; Health; *HealthEducation; Higher Education; *Laboratory ExFeriments; *Laboratory Techniques; *Laboratory Training;Medical Education; Pathology; Physics; *Physiology;*Science Education; Secondary Education IDENTIFIERS *Respiration ABSTItACT Designed to accompany the student text on respiration, this manual presents instructions on the use of labcra tcry equipment and presents various experimentsdealing with the concepts presented in the text. Thirty=nine laboratoryactivities ' are de scribed. Laboratory activities are dividedinto several parts, each Part covering a specific experiment dealing with the concept covered by the activity. Each experimentincludes a description of materials, procedures, and discurision questions. (RE) ********************************************************************** Reproductions supplied by EDRS are the bestthat can be made from the original document. 4********************M414141#***********4141*****4141***********41#********4 -
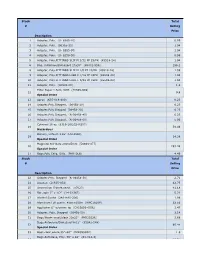
Stock Total # Selling Price 1 Adapter, Poly. (U- 6365-10)
Stock Total # Selling Price Description 1 Adapter, Poly. (U- 6365-10) 0.99 2 Adapter, Poly. (06365-22) 1.04 3 Adapter, Poly. (U- 6365-30) 1.04 4 Adapter, Poly. (U- 6359-50) 0.99 5 Adapter, Poly,FITTINGS SLIP M 3/32 PP 25/PK (45518-24) 1.04 6 Bag, Autoclave/Biohazard 24x30'' (95042-556) 156.1 8 Adapter, Poly.FITTINGS SLIP M 1/8 PP 25/PK (45518-26) 1.59 9 Adapter, Poly.FITTINGS LUER F 1/16 PP 25PK (45508-00) 1.04 10 Adapter, Poly.FITTINGS LUER F 3/32 PP 25PK (45508-02) 1.04 11 Adapter, Poly. (30800-08) 1.3 Filter Paper 1.5cm, VWR (28309-989) 12 9.9 Special Order 13 Apron (635-018-400) 0.23 14 Adapter,Poly.Stepped, (06458-10) 6.23 15 Adapter,Poly.Stepped (06458-20) 6.23 16 Adapter,Poly.Stepped, (N-06458-40) 6.23 17 Adapter,Poly.Stepped, (N-06458-60) 5.06 Cytoseal 16 oz (8310-16/23244257) 18 58.49 Hazardous Battery, Lithium 3.6V (LS14500) 19 14.38 Special Order Magnetic Stir Bars 2mmx5mm (58948-377) 20 293.48 Special Order 21 Bags,Poly Cello,10 lb. (PKR10Lb) 4.49 Stock Total # Selling Price Description 22 Adapter,Poly. Stepped (N-06458-30) 2.71 24 Alconox (21835-032) 42.75 25 Ammonium Sulfate,certif. (A7023) 41.18 26 Bar, spin 2'' x 1/2'' (14-51367) 5.22 27 Alcohol Swabs (248-HAS-200) 1.96 28 Aluminum Foil comm. 45cmx100m (WPC1810P) 32.56 29 Applicator 6'' w/cotton tip (CA10806-000L) 1.47 30 Adapter, Poly. -

Calorimetry Lab
P31220 lab Calorimetry Lab Purpose: Students will measure latent heat and specific heat. PLEASE READ the entire handout before starting. You won’t know what to do unless you understand how it works! Introduction: Calorimetry is the art of measuring energy. For example, determining how many calories are in a cheeseburger is done with a device called a “bomb calorimeter.” A sample of the food is burned in a closed container that is surrounded by water. The energy content of the food is determined from the temperature increase of the water jacket that surrounds the combustion chamber. In this lab, you will do two classic calorimetry experiments: measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Both experiments will use the same apparatus. Apparatus: Fig. 1 shows the construction of the basic calorimeter. The calorimeter is designed to minimize heat flow between the inner cup and the outside world. Conduction of heat is eliminated by supporting the inner cup only by the thin, insulating phenolic (a type of plastic) ring, and by providing an insulating air space around the cup. Convection is eliminated by blocking air circulation with the solid ring and the lid. Radiation is eliminated by making the inner cup and outer jacket out of aluminum, which is mirror- bright to infrared radiation. Fig. 1: The calorimeter. To use the calorimeter, the inner cup is half filled with a known mass of water, and the temperature is measured. The sample is added, the temperature is measured again, and the desired quantity (latent heat or specific heat) is calculated.