Gene Turnover in the Avian Globin Gene Families and Evolutionary Changes in Hemoglobin Isoform Expression Juan C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Giant Hummingbird from Paramo De Chingaza, Colombia.-On 10 October 1981, During an Ornithological Survey at 3250 M Elev
GENERAL NOTES 661 E. nigrivestis is timed to coincide with flowering of P. huigrensis (and other plants) which bloom seasonally at lower elevations. The general ecology of E. nigrivestis is similar to that recorded for the closely related species, the Glowing Puffleg (E. vextitus)(Snow and Snow 1980). Snow and Snow (1980) observed E. vestitus feeding principally at plants of similar general morphology (straight tubular corollas 10-20 mm in length) in similar taxonomic groups (Cnvenclishia; Ericaceae more often than Palicourea; Rubiaceae for E. vestitus). Territoriality in E. vestitus was pronounced while this was only suggested in the male E. nigrivestis we observed, perhaps a seasonal effect. Finally E. vestitus, like E. nigrivestis, did not occur in tall forested habitats at similar elevations (Snow and Snow [1980] study at 2400-2500 m). The evidence presented here suggests that the greatly restricted range of E. nigrivestis is not due to dietary specializations. Male nectar sources are from a broad spectrum of plants, and the principal food plant of both sexes, P. huigrensis, is a widespread Andean species. Nesting requirements of E. nigrivestis may still be threatened with extinction through habitat destruction, especially if the species requires specific habitats of natural vegetation at certain times of year, such as that found on nude ridge crests. The vegetation on nudes in particular is rapidly disappearing because nudes provide flat ground for cultivation in otherwise pre- cipitous terrain. In light of its restricted range and the threat of habitat destruction resulting from such close proximity to a major urban center, Quito, we consider E. -

Rare Birds of California Now Available! Price $54.00 for WFO Members, $59.99 for Nonmembers
Volume 40, Number 3, 2009 The 33rd Report of the California Bird Records Committee: 2007 Records Daniel S. Singer and Scott B. Terrill .........................158 Distribution, Abundance, and Survival of Nesting American Dippers Near Juneau, Alaska Mary F. Willson, Grey W. Pendleton, and Katherine M. Hocker ........................................................191 Changes in the Winter Distribution of the Rough-legged Hawk in North America Edward R. Pandolfino and Kimberly Suedkamp Wells .....................................................210 Nesting Success of California Least Terns at the Guerrero Negro Saltworks, Baja California Sur, Mexico, 2005 Antonio Gutiérrez-Aguilar, Roberto Carmona, and Andrea Cuellar ..................................... 225 NOTES Sandwich Terns on Isla Rasa, Gulf of California, Mexico Enriqueta Velarde and Marisol Tordesillas ...............................230 Curve-billed Thrasher Reproductive Success after a Wet Winter in the Sonoran Desert of Arizona Carroll D. Littlefield ............234 First North American Records of the Rufous-tailed Robin (Luscinia sibilans) Lucas H. DeCicco, Steven C. Heinl, and David W. Sonneborn ........................................................237 Book Reviews Rich Hoyer and Alan Contreras ...........................242 Featured Photo: Juvenal Plumage of the Aztec Thrush Kurt A. Radamaker .................................................................247 Front cover photo by © Bob Lewis of Berkeley, California: Dusky Warbler (Phylloscopus fuscatus), Richmond, Contra Costa County, California, 9 October 2008, discovered by Emilie Strauss. Known in North America including Alaska from over 30 records, the Dusky is the Old World Warbler most frequent in western North America south of Alaska, with 13 records from California and 2 from Baja California. Back cover “Featured Photos” by © Kurt A. Radamaker of Fountain Hills, Arizona: Aztec Thrush (Ridgwayia pinicola), re- cently fledged juvenile, Mesa del Campanero, about 20 km west of Yecora, Sonora, Mexico, 1 September 2007. -
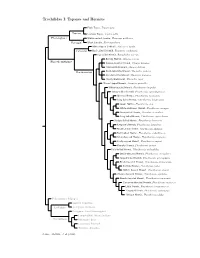
Topazes and Hermits
Trochilidae I: Topazes and Hermits Fiery Topaz, Topaza pyra Topazini Crimson Topaz, Topaza pella Florisuginae White-necked Jacobin, Florisuga mellivora Florisugini Black Jacobin, Florisuga fusca White-tipped Sicklebill, Eutoxeres aquila Eutoxerini Buff-tailed Sicklebill, Eutoxeres condamini Saw-billed Hermit, Ramphodon naevius Bronzy Hermit, Glaucis aeneus Phaethornithinae Rufous-breasted Hermit, Glaucis hirsutus ?Hook-billed Hermit, Glaucis dohrnii Threnetes ruckeri Phaethornithini Band-tailed Barbthroat, Pale-tailed Barbthroat, Threnetes leucurus ?Sooty Barbthroat, Threnetes niger ?Broad-tipped Hermit, Anopetia gounellei White-bearded Hermit, Phaethornis hispidus Tawny-bellied Hermit, Phaethornis syrmatophorus Mexican Hermit, Phaethornis mexicanus Long-billed Hermit, Phaethornis longirostris Green Hermit, Phaethornis guy White-whiskered Hermit, Phaethornis yaruqui Great-billed Hermit, Phaethornis malaris Long-tailed Hermit, Phaethornis superciliosus Straight-billed Hermit, Phaethornis bourcieri Koepcke’s Hermit, Phaethornis koepckeae Needle-billed Hermit, Phaethornis philippii Buff-bellied Hermit, Phaethornis subochraceus Scale-throated Hermit, Phaethornis eurynome Sooty-capped Hermit, Phaethornis augusti Planalto Hermit, Phaethornis pretrei Pale-bellied Hermit, Phaethornis anthophilus Stripe-throated Hermit, Phaethornis striigularis Gray-chinned Hermit, Phaethornis griseogularis Black-throated Hermit, Phaethornis atrimentalis Reddish Hermit, Phaethornis ruber ?White-browed Hermit, Phaethornis stuarti ?Dusky-throated Hermit, Phaethornis squalidus Streak-throated Hermit, Phaethornis rupurumii Cinnamon-throated Hermit, Phaethornis nattereri Little Hermit, Phaethornis longuemareus ?Tapajos Hermit, Phaethornis aethopygus ?Minute Hermit, Phaethornis idaliae Polytminae: Mangos Lesbiini: Coquettes Lesbiinae Coeligenini: Brilliants Patagonini: Giant Hummingbird Lampornithini: Mountain-Gems Tro chilinae Mellisugini: Bees Cynanthini: Emeralds Trochilini: Amazilias Source: McGuire et al. (2014).. -

Gene Duplication and the Evolution of Hemoglobin Isoform Differentiation in Birds
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Jay F. Storz Publications Papers in the Biological Sciences 11-2012 Gene Duplication and the Evolution of Hemoglobin Isoform Differentiation in Birds Michael T. Grispo University of Nebraska-Lincoln, [email protected] Chandrasekhar Natarajan University of Nebraska-Lincoln, [email protected] Joana Projecto-Garcia University of Nebraska–Lincoln Hideaki Moriyama University of Nebraska-Lincoln, [email protected] Roy E. Weber Aarhus University, Denmark See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/bioscistorz Grispo, Michael T.; Natarajan, Chandrasekhar; Projecto-Garcia, Joana; Moriyama, Hideaki; Weber, Roy E.; and Storz, Jay F., "Gene Duplication and the Evolution of Hemoglobin Isoform Differentiation in Birds" (2012). Jay F. Storz Publications. 61. https://digitalcommons.unl.edu/bioscistorz/61 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Jay F. Storz Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Michael T. Grispo, Chandrasekhar Natarajan, Joana Projecto-Garcia, Hideaki Moriyama, Roy E. Weber, and Jay F. Storz This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ bioscistorz/61 Published in Journal of Biological Chemistry 287:45 (November 2, 2012), pp. 37647-37658; doi: 10.1074/jbc.M112.375600 Copyright © 2012 The American Society for Biochemistry and Molecular Biology, Inc. -

Hummingbird (Family Trochilidae) Research: Welfare-Conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens
Special Publications Museum of Texas Tech University Number xx76 19xx January XXXX 20212010 Hummingbird (Family Trochilidae) Research: Welfare-conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens Lisa A. Tell, Jenny A. Hazlehurst, Ruta R. Bandivadekar, Jennifer C. Brown, Austin R. Spence, Donald R. Powers, Dalen W. Agnew, Leslie W. Woods, and Andrew Engilis, Jr. Dedications To Sandra Ogletree, who was an exceptional friend and colleague. Her love for family, friends, and birds inspired us all. May her smile and laughter leave a lasting impression of time spent with her and an indelible footprint in our hearts. To my parents, sister, husband, and children. Thank you for all of your love and unconditional support. To my friends and mentors, Drs. Mitchell Bush, Scott Citino, John Pascoe and Bill Lasley. Thank you for your endless encouragement and for always believing in me. ~ Lisa A. Tell Front cover: Photographic images illustrating various aspects of hummingbird research. Images provided courtesy of Don M. Preisler with the exception of the top right image (courtesy of Dr. Lynda Goff). SPECIAL PUBLICATIONS Museum of Texas Tech University Number 76 Hummingbird (Family Trochilidae) Research: Welfare- conscious Study Techniques for Live Hummingbirds and Processing of Hummingbird Specimens Lisa A. Tell, Jenny A. Hazlehurst, Ruta R. Bandivadekar, Jennifer C. Brown, Austin R. Spence, Donald R. Powers, Dalen W. Agnew, Leslie W. Woods, and Andrew Engilis, Jr. Layout and Design: Lisa Bradley Cover Design: Lisa A. Tell and Don M. Preisler Production Editor: Lisa Bradley Copyright 2021, Museum of Texas Tech University This publication is available free of charge in PDF format from the website of the Natural Sciences Research Laboratory, Museum of Texas Tech University (www.depts.ttu.edu/nsrl). -

Contents Contents
Traveler’s Guide WILDLIFE WATCHINGTraveler’s IN PERU Guide WILDLIFE WATCHING IN PERU CONTENTS CONTENTS PERU, THE NATURAL DESTINATION BIRDS Northern Region Lambayeque, Piura and Tumbes Amazonas and Cajamarca Cordillera Blanca Mountain Range Central Region Lima and surrounding areas Paracas Huánuco and Junín Southern Region Nazca and Abancay Cusco and Machu Picchu Puerto Maldonado and Madre de Dios Arequipa and the Colca Valley Puno and Lake Titicaca PRIMATES Small primates Tamarin Marmosets Night monkeys Dusky titi monkeys Common squirrel monkeys Medium-sized primates Capuchin monkeys Saki monkeys Large primates Howler monkeys Woolly monkeys Spider monkeys MARINE MAMMALS Main species BUTTERFLIES Areas of interest WILD FLOWERS The forests of Tumbes The dry forest The Andes The Hills The cloud forests The tropical jungle www.peru.org.pe [email protected] 1 Traveler’s Guide WILDLIFE WATCHINGTraveler’s IN PERU Guide WILDLIFE WATCHING IN PERU ORCHIDS Tumbes and Piura Amazonas and San Martín Huánuco and Tingo María Cordillera Blanca Chanchamayo Valley Machu Picchu Manu and Tambopata RECOMMENDATIONS LOCATION AND CLIMATE www.peru.org.pe [email protected] 2 Traveler’s Guide WILDLIFE WATCHINGTraveler’s IN PERU Guide WILDLIFE WATCHING IN PERU Peru, The Natural Destination Peru is, undoubtedly, one of the world’s top desti- For Peru, nature-tourism and eco-tourism repre- nations for nature-lovers. Blessed with the richest sent an opportunity to share its many surprises ocean in the world, largely unexplored Amazon for- and charm with the rest of the world. This guide ests and the highest tropical mountain range on provides descriptions of the main groups of species Pthe planet, the possibilities for the development of the country offers nature-lovers; trip recommen- bio-diversity in its territory are virtually unlim- dations; information on destinations; services and ited. -

Ecuador's Biodiversity Hotspots
Ecuador’s Biodiversity Hotspots Destination: Andes, Amazon & Galapagos Islands, Ecuador Duration: 19 Days Dates: 29th June – 17th July 2018 Exploring various habitats throughout the wonderful & diverse country of Ecuador Spotting a huge male Andean bear & watching as it ripped into & fed on bromeliads Watching a Eastern olingo climbing the cecropia from the decking in Wildsumaco Seeing ~200 species of bird including 33 species of dazzling hummingbirds Watching a Western Galapagos racer hunting, catching & eating a Marine iguana Incredible animals in the Galapagos including nesting flightless cormorants 36 mammal species including Lowland paca, Andean bear & Galapagos fur seals Watching the incredible and tiny Pygmy marmoset in the Amazon near Sacha Lodge Having very close views of 8 different Andean condors including 3 on the ground Having Galapagos sea lions come up & interact with us on the boat and snorkelling Tour Leader / Guides Overview Martin Royle (Royle Safaris Tour Leader) Gustavo (Andean Naturalist Guide) Day 1: Quito / Puembo Francisco (Antisana Reserve Guide) Milton (Cayambe Coca National Park Guide) ‘Campion’ (Wildsumaco Guide) Day 2: Antisana Wilmar (Shanshu), Alex and Erica (Amazonia Guides) Gustavo (Galapagos Islands Guide) Days 3-4: Cayambe Coca Participants Mr. Joe Boyer Days 5-6: Wildsumaco Mrs. Rhoda Boyer-Perkins Day 7: Quito / Puembo Days 8-10: Amazon Day 11: Quito / Puembo Days 12-18: Galapagos Day 19: Quito / Puembo Royle Safaris – 6 Greenhythe Rd, Heald Green, Cheshire, SK8 3NS – 0845 226 8259 – [email protected] Day by Day Breakdown Overview Ecuador may be a small country on a map, but it is one of the richest countries in the world in terms of life and biodiversity. -

WOMEN BIRDERS PROGRAM Sponsored By
© 2020 Jacamar Club - Perú WOMEN BIRDERS PROGRAM Sponsored by Jacamar Club and Vortex Optics A FIELD REPORT OF BIRDING EXCURSIONS AND THE IMPORTANCE OF EMPOWERING WOMEN IN THE BIRDING ACTIVITIES FOR ENVIROMENTAL EDUCATION IN PERU AND BOLIVIA LOCATION: PUKA PUKARA ARCHAEOLOGICAL CENTER, HUMEDAL DE HUAYLLARCOCHA AND SANTA MARIA ECOLOGICAL RESERVE. CUSCO - PERU JULY 11, 2020 [email protected] [email protected] www.JACAMARCLUB.com Redacted by: Ana Amable Page 1 Copyrights Jacamar Club 2020 © 2020 Jacamar Club - Perú The Women Birders Program is created basically to empower women in the good practice of birding & Birdwatching in Peru and Bolivia. Throutgh this program the women community will be able to increase their leadership and conservation skills, this will also make a difference in their communities and it will involved directly in the conservation of birds. The Jacamar Club programs perform a series of activities as a preliminary steps to environmental education, ecotourism ideas and conservation of bird habitats in Peru and Bolivia. Females birders at Puka Pukara Archaeological Center near Cusco city. Photo by Ana Amable. Page 2 Copyrights Jacamar Club 2020 © 2020 Jacamar Club - Perú BIRDING LOCALITIES OF INTEREST CUSCO Cuzco is a city in the Peruvian Andes that was the capital of the Inca Empire and is known for its archaeological remains and Spanish colonial architecture. The Plaza de Armas is the center of the old city, with galleries, carved wooden balconies and ruins of Inca walls. The Santo Domingo convent, in the Baroque style, was built on the Inca Sun Temple (Qoricancha) and has archaeological remains of Inca stonework. -

A Defence of Biodiversity As the Goal of Conservation Biology
A Defence of Biodiversity as the Goal of Conservation Biology Charles Gibson A thesis submitted for the degree of Doctor of Philosophy in Philosophy The University of Otago Dunedin New Zealand December 2018 i Dedication For my son Caelin, Who was born when this project began But was arguing with me by the time it was completed. May you have as much grass to run on as I did. ii Abstract Biodiversity has been the goal of conservation for thirty years but recent work by biodiversity eliminativists has raised serious challenges to its suitability as the primary goal of conservation. This project groups those challenges into three major arguments: the conceptual case for biodiversity’s elimination, the empirical case for biodiversity’s elimination, and the value compass case for biodiversity’s elimination. Aside from discussing biodiversity as a property, this thesis will also discuss biodiversity as a concept (as in biodiversity), and refer to the word biodiversity (as in ‘biodiversity’). In the conceptual case for biodiversity’s elimination, eliminativists argue that biodiversity misdirects the efforts of conservation and is not a scientifically coherent concept. In the empirical case, eliminativists argue that biodiversity is not operationalisable. In the value compass case, eliminativists argue that biodiversity does not reliably track biological value. I will argue that all three cases for biodiversity’s elimination are unsuccessful. Biodiversity is a complex concept with multiple dimensions of biological diversities but understanding it as a homeostatic property cluster avoids the conceptual case for its elimination. The empirical case is unsuccessful because the surrogacy strategy for measuring biodiversity can be defended against its limitations and the expanding multiplicity of biodiversity measures is overblown. -

Mexican Violetear 07-12-16
LOUISIANA BIRD RECORDS COMMITTEE REPORT FORM This form is intended as a convenience in reporting observations of species on the Louisiana Bird Records Committee (LBRC) Review List. The LBRC recommends the use of this form or a similar format when submitting records for review to assure that all pertinent information is accounted for. Attach additional pages or files as necessary. Please print or type for hard copy. For electronic copy, be sure to save this file to your computer before entering text. Attach field notes, drawings, photographs, or tape recordings, if available. Include all photos for more obscurely marked species. When completed (if hard copy), mail to Secretary, Louisiana Bird Records Committee, c/o Museum of Natural Science, 119 Foster Hall, Louisiana State University, Baton Rouge, LA 70803-3216, or e-mail electronic copy as an attachment to Paul Edward Conover at <[email protected]> . 1. English and Scientific names: Mexican Violetear; Colibri thalassinus 2. Number of individuals, sexes, ages, general plumage (e.g., 2 in alternate plumage): !1, possibly immature 3. Parish: Bienville Parish Specific Locality: 2624 Jot Em Down Drive, Arcadia 4. Date(s) when observed: 11-12 July, 2016 (seen by homeowner first on 10 July; last seen on 14 July) ! 5. Time(s) of day when observed: 11 July: 7:30 - 8:15pm. 12 July: 5:50 - 10:00am. ! 6. Reporting observer and address: John Dillon 183 Dance Road, Athens, LA ! 7. Other observers accompanying reporter who also identified the bird(s): homeowner, Donna Butler. ! 8. Other observers who independently identified the bird(s): Charlie Lyon and Rosemary Seidler both visited separately and saw the bird. -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed -

1 Husbandry Guidelines Apodiformes Hummingbirds-Trochilidae Karen
Husbandry Guidelines Apodiformes Hummingbirds-Trochilidae Karen Krebbs, Conservation Biologist / Arizona-Sonora Desert Museum / Tucson, AZ Dave Rimlinger, Curator of Ornithology / San Diego Zoo / San Diego, CA Michael Mace, Curator of Ornithology / San Diego Wild Animal Park / Escondido, CA September, 2002 1. ACQUISITION AND ACCLIMATIZATION Sources of birds & acclimatization procedures - In the United States local species of hummingbirds can be collected with the proper permits. The Arizona-Sonora Desert Museum usually has species such as Anna's (Calypte anna), Costa's (Calypte costae), and Broad-billed (Cynanthus latirostris) for surplus each year if these species have nested in their Hummingbird Exhibit. In addition to keeping some native species, the San Diego Zoo has tried to maintain several exotic species such as Sparkling violet-ear (Colibri coruscans), Emerald (Amazilia amazilia), Oasis (Rhodopis vesper), etc. The San Diego Wild Animal Park has a large mixed species glass walk-through enclosure and has kept and produced hummingbirds over the years. All hummingbirds are on Appendix II of CITES and thus are covered under the Wild Bird Conservation Act (WBCA). An import permit from USFWS and an export permit from the country of origin must be obtained prior to the importation. Permits have been granted in the past, but currently it is difficult to find a country willing to export hummingbirds. Hummingbirds are more commonly kept in European collections, particularly private collections, and could be a source for future imports. Weighing Hummingbirds can be placed in a soft mesh bag and weighed with a spring scale. Electronic digital platform scales can also be used. A small wooden crate with a wire mesh front can also be used for weighing.