Atomic Weights of the Elements 2013 (IUPAC Technical Report)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gadolinium Information
Gadolinium Information Gadolinium contrast agents are frequently utilized during MRI examinations in order to improve the exam and interpretation. They are not always needed. Your radiologist will determine whether or not gadolinium contrast is needed for your MRI examination. Gadolinium contrast agents are quickly eliminated from the body in healthy individuals. With normal functioning kidneys, the retention of gadolinium in soft tissues of the body is very small and may not even be detectable. However, some patients who receive multiple doses of contrast, including pregnant women and children, might be at increased risk of gadolinium remaining in the body for longer periods of time. To date, there are no known harmful effects of gadolinium remaining in the body for long periods of time in patients who have normal kidneys. In patients who have poorly functioning kidneys, a condition called nephrogenic systemic sclerosis (NSF) can occur. This causes debilitating thickening of the skin and other tissues. This only occurs in patients with poorly functioning kidneys. Your kidney function will be checked prior to receiving gadolinium contrast agent if needed. Other side-effects can occur even in patients with healthy kidneys. Some patients report pain, tiredness, and muscle aches after receiving gadolinium contrast but these conditions have not been directly linked to the administration of the gadolinium. Allergic reactions can also occur, as with any drug. If you have questions regarding your MRI examination today, please ask your MRI Technologist. MEDICATION GUIDE MULTIHANCE® (məl-tē-han(t)s) (gadobenate dimeglumine) Injection for intravenous use What is MULTIHANCE? • MULTIHANCE is a prescription medicine called a gadolinium-based contrast agent (GBCA). -

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -

Synthesis and Characterization of the New Uranium Yttrium Oxysulfide
ARTICLE IN PRESS Journal of Solid State Chemistry 182 (2009) 1861–1866 Contents lists available at ScienceDirect Journal of Solid State Chemistry journal homepage: www.elsevier.com/locate/jssc Synthesis and characterization of the new uranium yttrium oxysulfide UY4O3S5 Geng Bang Jin a, Eun Sang Choi b, Daniel M. Wells a, James A. Ibers a,Ã a Department of Chemistry, Northwestern University, 2145 Sheridan Road, Evanston, IL 60208-3113, USA b Department of Physics and National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL 32310, USA article info abstract Article history: Red needles of UY4O3S5 have been synthesized by the solid-state reaction at 1273 K of UOS and Y2S3 Received 16 January 2009 with Sb2S3 as a flux. UY4O3S5 adopts a three-dimensional structure that contains five crystal- Received in revised form lographically unique heavy-atom positions. U and Y atoms disorder on one eight-coordinate metal 11 April 2009 position bonded to four O atoms and four S atoms and two seven-coordinate metal positions bonded to Accepted 20 April 2009 three O atoms and four S atoms. Another eight-coordinate metal position with two O and six S atoms Available online 3 May 2009 and one six-coordinate metal position with six S atoms are exclusively occupied by Y atoms. UY4O3S5 is Keywords: a modified Curie–Weiss paramagnet between 1.8 and 300 K. Its effective magnetic moment is estimated Uranium yttrium oxysulfide to be 3.3(2) mB.UY4O3S5 has a band gap of 1.95 eV. The electrical resistivity along the [010] direction of a X-ray crystal structure single crystal shows Arrhenius-type thermal activation with an activation energy of 0.2 eV. -
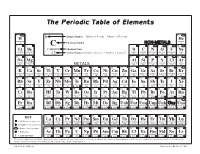
The Periodic Table of Elements
The Periodic Table of Elements 1 2 Atomic Number = Number of Protons = Number of Electrons H 6 He HYDROGEN HELIUM 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM Atomic Weight = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 11 12 14 16 19 20 11 12 13 14 15 16 17 18 Na Mg Al Si P S Cl Ar SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn CESIUM BARIUM HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 Fr Ra Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo FRANCIUM RADIUM -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Darmstadtium, Roentgenium and Copernicium Form Strong Bonds with Cyanide
Darmstadtium, Roentgenium and Copernicium Form Strong Bonds With Cyanide Taye B. Demissie∗and Kenneth Ruudy March 23, 2017 Abstract We report the structures and properties of the cyanide complexes of three super- heavy elements (darmstadtium, roentgenium and copernicium) studied using two- and four-component relativistic methodologies. The electronic and structural properties of these complexes are compared to the corresponding complexes of platinum, gold and mercury. The results indicate that these superheavy elements form strong bonds with cyanide. Moreover, the calculated absorption spectra of these superheavy-element cyanides show similar trends to those of the corresponding heavy-atom cyanides. The calculated vibrational frequencies of the heavy-metal cyanides are in good agreement with available experimental results lending support to the quality of our calculated vibrational frequencies for the superheavy-atom cyanides. ∗Centre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway yCentre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic Uni- versity of Norway, N-9037 Tromsø, Norway 1 Relativistic two- and four-component density-functional theory is used to demonstrate that the superheavy elements darmstadtium, roentgenium and copernicium form stable complexes with cyanide, providing new insight into the chemistry of these superheavy elements. 2 INTRODUCTION The term heavy atom refers roughly to elements in the 4th - -

Gadolinium Speciation
Gadolinium Speciation Peter Caravan Martinos Center for Biomedical Imaging Institute for Innovation in Imaging Massachusetts General Hospital and Harvard Medical School Conflicts of Interest Stock ownership (>5%): Reveal Pharmaceuticals; Collagen Medical; Factor 1A LLC. Research grants: Pfizer; Pliant Pharmaceuticals; Biogen; Agilent; Pharmakea; Siemens. Consulting: Guerbet; Bayer; Collagen Medical; UCB Biopharma; Pfizer. What do we mean by speciation? What is the chemical form of the gadolinium in tissue? Chelated Gd Dissociated Gd The GBCA remains intact Dissociation of the GBCA Gd3+ ion Is Gd bound to a low molecular weight ligand? Is Gd part of some inorganic material like hydroxyapatite? Is Gd bound to a macromolecule? If so, which one? Where is the Gd distributed within tissue? Extra vs intracellular? In which cellular compartments? Why do we care about speciation? • The chemical form of Gd may inform its potential toxicity • Mineralized, insoluble Gd may be less toxic than soluble protein bound Gd (hypothesis) • The chemical form may also inform whether the Gd will be ultimately eliminated. Intact chelate may be expected to eventually clear the body (hypothesis). • The chemical form and location may guide chelation therapy strategies. Hierarchy of relevance of the data Human in vivo Human ex vivo Animal in vivo Animal ex vivo Solutions To model ex vivo Water solutions Tweedle MF. Gadolinium deposition: Is it chelated or dissociated gadolinium? How can we tell? Magn Reson Imaging. 2016;34(10):1377–82. How do GBCAs differ • Thermodynamics: -

Uptake of Nanoparticles of Cerium Oxide and Yttrium Oxide by Acanthamoeba Castellanii (Protozoa) and Daphnia Magna (Crustacea) James R
Virginia Journal of Science Volume 62, 1 & 2 Spring 2011 Uptake of Nanoparticles of Cerium Oxide and Yttrium Oxide by Acanthamoeba castellanii (Protozoa) and Daphnia magna (Crustacea) James R. Palmieri1, Geneva Gehring, Catherine Minichino, and Shaadi F. Elswaifi Department of Microbiology, Infectious, and Emerging Diseases, Edward Via College of Osteopathic Medicine, 2265 Kraft Drive, Blacksburg, Virginia, USA 24060 ABSTRACT Currently, nanoparticles are synthesized and used at an unprecedented rate for industrial, medical, and research applications. The use of cerium oxide nanoparticles (CeONP) and yttrium oxide nanoparticles (YtONP) results in their spread as contaminants into the environment. Once in the environment, CeONP and YtONP can be taken up by organisms in the food chain where they may pose a public health risk. In this study we determine whether Acanthamoeba castellanii and Daphnia magna uptake CeONP or YtONP from their environment and thereby play a role in the transmission of the nanoparticles. Using electron microscopy, organisms exposed to the nanoparticles were examined. Our results indicate that the nanoparticles are associated with cell and organelle membranes. These findings have implications for the health risks associated with environmental contamination by CeONP and YtONP. INTRODUCTION In this study we determine whether protists and crustaceans play a role in the transfer of cerium oxide nanoparticles (CeONP) and yttrium oxide nanoparticles (YtONP) from the environment to other organisms within the aquatic food chain. Acanthamoeba castellanii, a common protist, and Daphnia magna, a planktonic crustacean, are important components in many aquatic ecosystems. Because acanthamoebae, such as A. castellanni, are aggressive feeders they consume inorganic and organic compounds from their environment, thereby serving as a link by transferring normally unavailable inorganic components to the food chain (Weekers et al. -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

Diffusion of Carbon in Niobium and Molybdenum
Materials Transactions, Vol. 55, No. 12 (2014) pp. 1786 to 1791 ©2014 The Japan Institute of Metals and Materials Diffusion of Carbon in Niobium and Molybdenum Jun-ichi Imai1, Osamu Taguchi2,+, Gyanendra Prasad Tiwari3 and Yoshiaki Iijima1 1Department of Materials Science, Graduate School of Engineering, Tohoku University, Sendai 980-8579, Japan 2Department of Materials Science and Engineering, Miyagi National College of Technology, Natori 981-1239, Japan 3Department of Information Technology, Ramrao Adik Institute of Technology, Vidya Nagri, Nerul, Navi Mumbai 400709, India Diffusion coefficients of carbon in niobium and molybdenum have been determined by the residual activity method with radioactive tracer 14C in the temperature ranges between 1168 and 1567 K for niobium and between 1271 and 1669 K for molybdenum. The temperature dependences of the diffusion coefficient of carbon in niobium and molybdenum are expressed by D/m2 s¹1 = 2.2 © 10¹6 exp(¹152 kJ mol¹1/ RT) and D/m2 s¹1 = 5.2 © 10¹6 exp(¹163 kJ mol¹1/RT), respectively. Since the solubility of carbon in molybdenum is very small, the diffusion of carbon in molybdenum is strongly influenced by carbide precipitation at lower temperatures. [doi:10.2320/matertrans.M2014277] (Received July 31, 2014; Accepted September 30, 2014; Published November 8, 2014) Keywords: carbon diffusion, niobium, molybdenum, carbon solubility, precipitate effect 1. Introduction 2. Experimental Procedure Iron, nickel and cobalt based superalloys appear to have 2.1 Material achieved full potential in relation to their use as structural Niobium metal rod arc-melted and machined to 12.5 mm in materials for corrosive environments as well as high diameter was supplied by Materials Research Corporation, temperatures.1) The strength of these alloys comes partly USA. -

Concurrent Reduction and Distillation; an Improved Technique for the Recovery and Chemical Refinement of the Isotopes of Cadmium and Zinc*
If I CONCURRENT REDUCTION AND DISTILLATION; AN IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC* H. H. Cardill L. E. McBride LOHF-821046—2 E. W. McDaniel DE83 001931 Operations Division Oak Ridge National Laboratory Oak Ridge, Tennessee 3 7830 .DISCLAIMER • s prepared es an accouni oi WOTk sconsQ'ed by an agency of itie United Slates Cover r "iiit?3 Stilt 65 Gov(?fnft»gn ^ ri(jt ^nv aQcncy Tllf GO* ^or rlny O^ tt^^ir ^rnployut^. TigV MASTER lostifl. ion, apod'aiuj pioiJut;. <y vned nghtv Reference hcein xc any v>t^-'T*t. ^ Dv ^.r^de narno, tf^Oernafk^ mir'ij'iJClureT. or otrierw «d <Jo*^ •nenaalion. or liivONng bv 'Vhe United _^_ . vs and opinions of authors enp'«i«] herein do noi IOM t' irip UnitK* Stales Govefnrneni c a^v agency For presentation at the 11th World Conference of the International Nuclear Target Development Society, October 6-8, 1982, Seattle, Washington. By acceptance of this article, the publisher or recipient acknowledge* the U.S. Govarnment's right to retain a nonaxclusiva, royalty-frtta license in and to any copyright covering tha article. NOTICE P0T09_0£-Jfns,J^SPQRT_JfgE_Il,LESIBLE. I has been reproauce.1 from the best available aopy to pensit the broadest possible avail- ability* *Research sponsored by the Office of Basic Energy Science, U.S. Department of Energy under contract W-7405-eng-26 with the Union Carbide Co rpcration,, DISTRIBUTION OF THIS DOCUMEHT 18 UNLIMITED ' CONCURRENT REDUCTION AND DISTILLATION - AY. IMPROVED TECHNIQUE FOR THE RECOVERY AND CHEMICAL REFINEMENT OF THE ISOTOPES OF CADMIUM AND ZINC H. -

Cerium Oxide Nanoparticles and Gadolinium Integration
Linköping Studies in Science and Technology Dissertation No. 1997 Peter Eriksson Peter FACULTY OF SCIENCE AND ENGINEERING Linköping Studies in Science and Technology, Dissertation No. 1997, 2019 Cerium Oxide Nanoparticles Department of Physics, Chemistry and Biology (IFM) Linköping University SE-581 83 Linköping, Sweden and Gadolinium Integration Cerium Oxide Integration Nanoparticles and Gadolinium Synthesis, Characterization and Biomedical Applications www.liu.se Peter Eriksson 2019 Linköping Studies in Science and Technology Dissertation No. 1997 Cerium Oxide Nanoparticles and Gadolinium Integration Synthesis, Characterization and Biomedical Applications Peter Eriksson Applied Physics Department of Physics, Chemistry & Biology Linköping University, Sweden Linköping 2019 Front cover: A cerium oxide nanoparticle with integrated gadolinium. Back cover: Cross-section of a gadolinium-cerium oxide nanoparticle and its displayed theragnostic properties: left) cerium undergo redox-reactions to scavenge reactive oxygen species and right) gadolinium shorten the T1-relaxation time of nuclei spins in water molecules. During the course of the research underlying this thesis, Peter Eriksson was enrolled in Forum Scientium, a multidisciplinary doctoral programme at Linköping University, Sweden. © Copyright 2019 Peter Eriksson, unless otherwise noted Peter Eriksson Cerium Oxide Nanoparticles and Gadolinium Integration; Synthesis, Characterization and Biomedical Applications ISBN: 978-91-7685-029-9 ISSN: 0345-7524 Linköping Studies in Science and Technology,