Chemistry of the Atmosphere-Foundation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atmospheric Pressure
Atmospheric pressure We all know that the atmosphere of Earth exerts a pressure on all of us. This pressure is the result of a column of air bearing down on us. However, in the seventeenth century, many scientists and philosophers believed that the air had no weight, which we already proved to be untrue in the lab (Remembered the fun you had sucking air out of the POM bottle?). Evangelista Torricelli, a student of Galileo’s, proved that air has weight using another experiment. He took a glass tube longer than 760 mm that is closed at one end and filled it completely with mercury. When he inverted the tube into a dish of mercury, some of the mercury flows out, but a column of mercury remained inside the tube. Torricelli argued that the mercury surface in the dish experiences the force of Earth’s atmosphere due to gravity, which held up the column of mercury. The force exerted by the atmosphere, which depends on the atmospheric pressure, equals the weight of mercury column in the tube. Therefore, the height of the mercury column can be used as a measure of atmospheric pressure. Although Torricelli’s explanation met with fierce opposition, it also had supporters. Blaise Pascal, for example, had one of Torricelli’s barometers carried to the top of a mountain and compared its reading there with the reading on a duplicate barometer at the base of the mountain. As the barometer was carried up, the height of the mercury column decreased, as expected, because the amount of air pressing down on the mercury in the dish decreased as the instrument was carried higher. -

Characterising the Three-Dimensional Ozone Distribution of a Tidally Locked Earth-Like Planet
Proedrou and Hocke Earth, Planets and Space (2016) 68:96 DOI 10.1186/s40623-016-0461-x FULL PAPER Open Access Characterising the three‑dimensional ozone distribution of a tidally locked Earth‑like planet Elisavet Proedrou1,2*† and Klemens Hocke1,2,3† Abstract We simulate the 3D ozone distribution of a tidally locked Earth-like exoplanet using the high-resolution, 3D chemistry- climate model CESM1(WACCM) and study how the ozone layer of a tidally locked Earth (TLE) (�TLE = 1/365 days) differs from that of our present-day Earth (PDE) �( PDE = 1/1 day). The middle atmosphere reaches a steady state asymptotically within the first 80 days of the simulation. An upwelling, centred on the subsolar point, is present on the day side while a downwelling, centred on the antisolar point, is present on the night side. In the mesosphere, we find similar global ozone distributions for the TLE and the PDE, with decreased ozone on the day side and enhanced ozone on the night side. In the lower mesosphere, a jet stream transitions into a large-scale vortex around a low-pressure system, located at low latitudes of the TLE night side. In the middle stratosphere, the concentration of odd oxygen is approximately equal to that of the ozone [(Ox) ≈ (O3)]. At these altitudes, the lifetime of odd oxygen is ∼16 h and the transport processes significantly contribute to the global distribution of stratospheric ozone. Compared to the PDE, where the strong Coriolis force acts as a mixing barrier between low and high latitudes, the transport processes of the TLE are governed by jet streams variable in the zonal and meridional directions. -

What Is Ozone Layer? a Layer in the Atmosphere of Earth That Protects Us from Harmful UV Rays from the Sun
International Day for Preservation of Ozone Layer 16 September 2020 Image Source: https://sfxstl.org/circleofcreation What is Ozone layer? A layer in the atmosphere of Earth that protects us from harmful UV rays from the Sun. It’s responsible for preserving life on the planet! What is happening? The ozone layer has begun to deplete because of harmful chemical substances and gases. This problem is not only contributing to global warming and climate change, but also allowing the dangerous radiation from the sun to affect human beings and ecosystems! Source: https://www.un.org/en/events/ozoneday/ What is causing its depletion? ● Human activities are the biggest cause of the ozone layer depletion ● When we burn coal, natural gas and other fuels for electricity, they release harmful gases such as carbon dioxide, nitrous oxide, etc that spread into the atmosphere and surround us like a blanket ● These harmful gases, called greenhouse gases, trap heat and radiation from the sun, which is causing the depletion as well as global warming. Clorofluorocarbons (CFCs), which are found in ACs and halogens, are other harmful greenhouse gases. Sources: https://e360.yale.edu/features/geoengineer-the-planet-more-scientists-now-say-it-must-be-an-option https://www.ucsusa.org/resources/ozone-hole-and-global-warming What can we do? Some things we can do to reduce our contribution to ozone layer depletion are: 1. Minimize use of cars. 2. Maintain your ACs regularly. 3. Avoid using products that are harmful to the environment and to us. 4. Buy local products which are more eco- friendly. -

A Study on the Concentration and Dispersion of Pm10 in Uthm by Using Simple Modelling and Meteorological Factors
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UTHM Institutional Repository i A STUDY ON THE CONCENTRATION AND DISPERSION OF PM10 IN UTHM BY USING SIMPLE MODELLING AND METEOROLOGICAL FACTORS MALEK FAIZAL B ABD RAHMAN A Project Report submitted in partial fulfilment of the requirements for the award of the Degree of Master of Engineering in Civil Engineering Faculty of Civil and Environmental Engineering Universiti Tun Hussein Onn Malaysia FEBRUARY, 2013 v ABSTRACT Air pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere. Air pollution can also be known as degradation of air quality resulting from unwanted chemicals or other materials occurring in the air. The simple way to know how polluted the air is to calculate the amounts of foreign and/or natural substances occurring in the atmosphere that may result in adverse effects to humans, animals, vegetation and/or materials. The objective of this study is to create a simulation of air quality dispersion in UTHM campus by using computer aided design mechanism such as software and calculating tools. Another objective is to compare the concentration obtained from the end result of calculation with past studies. The air pollutant in the scope of study is Particulate Matter (PM10). The highest reading recorded for E-Sampler was 305µg/m3. It was recorded in Structure Lab sampling point while the highest expected concentration by the Gaussian Dispersion Model was 184µg/m3 for UTHM Stadium. -
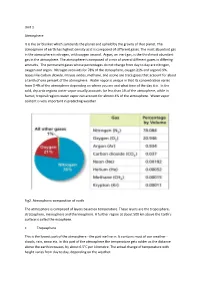
Unit 1 Atmosphere It Is the Air Blanket Which Surrounds the Planet And
Unit 1 Atmosphere It is the air blanket which surrounds the planet and upheld by the gravity of that planet. The atmosphere of earth has highest density as it is composed of different gases. The most abundant gas in the atmosphere is nitrogen, with oxygen second. Argon, an inert gas, is the third most abundant gas in the atmosphere. The atmosphere is composed of a mix of several different gases in differing amounts. The permanent gases whose percentages do not change from day to day are nitrogen, oxygen and argon. Nitrogen accounts for 78% of the atmosphere, oxygen 21% and argon 0.9%. Gases like carbon dioxide, nitrous oxides, methane, and ozone are trace gases that account for about a tenth of one percent of the atmosphere. Water vapor is unique in that its concentration varies from 0-4% of the atmosphere depending on where you are and what time of the day it is. In the cold, dry artic regions water vapor usually accounts for less than 1% of the atmosphere, while in humid, tropical regions water vapor can account for almost 4% of the atmosphere. Water vapor content is very important in predicting weather. Fig2. Atmospheric composition of earth The atmosphere is comprised of layers based on temperature. These layers are the troposphere, stratosphere, mesosphere and thermosphere. A further region at about 500 km above the Earth's surface is called the exosphere. • Troposphere This is the lowest part of the atmosphere - the part we live in. It contains most of our weather - clouds, rain, snow etc. In this part of the atmosphere the temperature gets colder as the distance above the earth increases, by about 6.5°C per kilometre. -

6Th Grade SDP Science Teachers
Curriculum Guide for 6th Grade SDP Science Teachers Please note: Pennsylvania & Next Generation Science Standards as well as Instructional Resources are found on the SDP Curriculum Engine Prepared by :Emily McGady, Science Curriculum Specialist 8/2017 1 6th Grade (Earth) Science Curriculum Term 1 (9/5-11/13/17) Topic: Landforms Duration: 9-10 Weeks Performance Objectives SWBAT: • analyze models of Earth’s various landforms (e.g., mountains, peninsulas) IOT identify and describe these landforms. • compare and contrast different bodies of water on Earth (e.g., streams, ponds, lakes, creeks) IOT categorize water systems as lentic or lotic. • compare and contrast different water systems (e.g., wetlands, oceans, rivers, watersheds) IOT describe their relationship to each other as well as to landforms. • create a stream table IOT explore relationships between systems, water, and land. • identify features of maps and diagrams IOT interpret what models represent. • describe Earth’s natural processes IOT analyze their effects on the Earth’s systems. • give examples of weathering and erosion IOT describe the impacts of weathering and erosion on landforms. • construct a scientific explanation based on evidence IOT determine how the uneven distributions of Earth’s mineral, energy, and groundwater resources are the result of past and current geoscience processes. • construct an explanation based on evidence IOT determine how geoscience processes have changed Earth’s surface at varying time and spatial scales. Define the criteria and constraints of a design problem IOT provide sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions. -

Air Pollution
Annals of Agricultural and Environmental Medicine 2019, Vol 26, No 4, 566–571 www.aaem.pl ORIGINAL ARTICLE Air pollution: how many cigarettes does each Pole ‘smoke’ every year and how does it influence health, with special respect to lung cancer? Robert Chudzik1,A-D , Paweł Rybojad1,2,C-F , Katarzyna Jarosz-Chudzik3,B-C , Marek Sawicki1,E-F , Beata Rybojad4,5,C,E-F , Lech Panasiuk6,E-F 1 Chair and Department of Thoracic Surgery, Medical University, Lublin, Poland 2 Department of Thoracic Surgery, Holy Cross Cancer Centre, Kielce, Poland 3 Independent Public Clinical Hospital No. 4, Lublin, Poland 4 Department of Emergency Medicine, Medical University, Lublin, Poland 5 Department of Anaesthesiology and Intensive Care, Children’s University Hospital, Lublin, Poland 6 Institute of Rural Health, Lublin, Poland A – Research concept and design, B – Collection and/or assembly of data, C – Data analysis and interpretation, D – Writing the article, E – Critical revision of the article, F – Final approval of article Chudzik R, Rybojad P, Jarosz-Chudzik K, Sawicki M, Rybojad B, Panasiuk L. Air pollution: how many cigarettes does each Pole ‘smoke’ every year and how does it influence health, with special respect to lung cancer? Ann Agric Environ Med. 2019; 26(4): 566–571. doi: 10.26444/aaem/109974 Abstract Introduction. Air pollution is one of the most important issues of our times. Air quality assessment is based on the measurement of the concentration of substances formed during the combustion process and micro-particles suspended in the air in the form of an aerosol. Microscopic atmospheric particulate matters (PM) 2.5 and 10 are mixtures of organic and inorganic pollutants smaller than 2.5 and 10 µm, respectively. -

Photochemical Smog
ATMOSPHERE The atmosphere is a thin protective blanket that nurtures life on Earth and protects it from the hostile environment of outer space by absorbing energy and damaging ultraviolet radiation from the sun and by moderating the Earth’s temperature to within a range conducive to life. The Atmosphere of Earth is Composed of : Gas % Nitrogen 78.08 Oxygen 20.95 Argon 0.93 Carbon dioxide 0.0365 In addition, air contains trace amounts of neon, helium, methane, krypton, nitrous oxide, hydrogen, xenon, sulfur dioxide, ozone, nitrogen dioxide, ammonia, carbon monoxide as well as water vapor. Layers of the Atmosphere : Exosphere Thermosphere The atmosphere is composed of discrete layers. Atoms and molecules travel rapidly Mesosphere within a layer but only very slowly between Stratosphere layers. The layering results from temperature Troposphere variations of the gas molecules. i. Troposphere The troposphere is the lowest layer of our atmosphere. Starting at ground level, it extends upward to about 10 km above sea level. Humans live in the troposphere, and nearly all weather occurs in this lowest layer. Most clouds appear here, mainly because 99% of the water vapor in the atmosphere is found in the troposphere. Air pressure drops, and temperatures get colder, as you climb higher in the troposphere. ii. Stratosphere Stratosphere is the second layer of the Earth's atmosphere. The stratosphere extends from the top of the troposphere to about 50 km above the ground. The infamous ozone layer is found within the stratosphere. Ozone molecules in this layer absorb high-energy ultraviolet (UV) light from the Sun, converting the UV energy into heat. -

The Carbon Cycle
TEACHER GUIDE THE CARBON CYCLE MATERIALS PER GROUP: LESSON OVERVIEW: Activity 1 The carbon cycle is the biogeochemical cycle by which carbon is • Carbonated water (clear soda or exchanged among the biosphere, pedosphere, geosphere, hydro- seltzer water) sphere and atmosphere of Earth. Along with the nitrogen cycle • Hot water (50°C) and the water cycle, the carbon cycle comprises a sequence of • Cold water (5°C) events key to making Earth capable of sustaining life; it describes • Two 12 oz. plastic bowls the movement of carbon as it is recycled and reused throughout • Two small (3 oz.) clear plastic cups the biosphere. Activity 2 LESSON OBJECTIVES: • Vinegar Students will be able to: • Baking soda 1. Observe the density of carbon dioxide. • Universal indicator 2. Demonstrate the effect of temperature on the amount of car- • Water bon dioxide that will dissolve in water. • Water and indicator cup 3. Test the effect of carbon dioxide on the pH of water. • Baking soda and vinegar 4. Describe how these properties of carbon dioxide relate to the • Mini spoon carbon cycle. • Universal indicator pH color chart ESSENTIAL QUESTION: Activity 3 How does matter cycle through an ecosystem? • Bubble generator* • Dry ice (8 oz.) TOPICAL ESSENTIAL QUESTION: • 9 oz. plastic cup What is carbon’s role in life on Earth? • Water (enough to fill bubble generator half-full) • Plastic spoon TOTAL DURATION: • Dish soap (5 mL) 15-20 min. pre-lab prep time; 40-50 min. class time • Cotton glove SAFETY PRECAUTIONS: *A bubble generator can be purchased from • Avoid contact of all chemicals with eyes and skin. -

Mars: Life, Subglacial Oceans, Abiogenic Photosynthesis, Seasonal Increases and Replenishment of Atmospheric Oxygen
Open Astron. 2020; 29: 189–209 Review Article Rhawn G. Joseph*, Natalia S. Duxbury, Giora J. Kidron, Carl H. Gibson, and Rudolph Schild Mars: Life, Subglacial Oceans, Abiogenic Photosynthesis, Seasonal Increases and Replenishment of Atmospheric Oxygen https://doi.org/10.1515/astro-2020-0020 Received Sep 3, 2020; peer reviewed and revised; accepted Oct 12, 2020 Abstract: The discovery and subsequent investigations of atmospheric oxygen on Mars are reviewed. Free oxygen is a biomarker produced by photosynthesizing organisms. Oxygen is reactive and on Mars may be destroyed in 10 years and is continually replenished. Diurnal and spring/summer increases in oxygen have been documented, and these variations parallel biologically induced fluctuations on Earth. Data from the Viking biological experiments also support active biology, though these results have been disputed. Although there is no conclusive proof of current or past life on Mars, organic matter has been detected and specimens resembling green algae / cyanobacteria, lichens, stromatolites, and open apertures and fenestrae for the venting of oxygen produced via photosynthesis have been observed. These life-like specimens include thousands of lichen-mushroom-shaped structures with thin stems, attached to rocks, topped by bulbous caps, and oriented skyward similar to photosynthesizing organisms. If these specimens are living, fossilized or abiogenic is unknown. If biological, they may be producing and replenishing atmospheric oxygen. Abiogenic processes might also contribute to oxygenation via sublimation and seasonal melting of subglacial water-ice deposits coupled with UV splitting of water molecules; a process of abiogenic photosynthesis that could have significantly depleted oceans of water and subsurface ice over the last 4.5 billion years. -
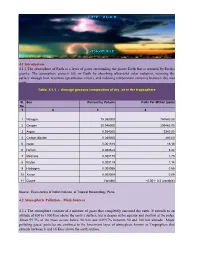
Main Sources 4.2.1 the Atmosphere Consists of a Mixture of Gases That Completely Surround the Eart
4.1 Introduction 4.1.1 The atmosphere of Earth is a layer of gases surrounding the planet Earth that is retained by Earth's gravity. The atmosphere protects life on Earth by absorbing ultraviolet solar radiation, warming the surface through heat retention (greenhouse effect), and reducing temperature extremes between day and night. Table 4.1.1 : Average gaseous composition of dry air in the troposphere Sl. Gas Percent by Volume Parts Per Million (ppm) No. 1 2 3 4 1 Nitrogen 78.080000 780840.00 2 Oxygen 20.946000 209460.00 3 Argon 0.934000 9340.00 4 Carbon dioxide 0.039000 390.00 5 Neon 0.001818 18.18 6 Helium 0.000524 5.24 7 Methane 0.000179 1.79 8 Kryton 0.000114 1.14 9 Hydrogen 0.000055 0.55 10 Xenon 0.000009 0.09 11 Ozone Variable ~0.001- 0.3 (variable) Source : Envis centre of Indian Institute of Tropical Meteorology, Pune. 4.2 Atmospheric Pollution – Main Sources 4.2.1 The atmosphere consists of a mixture of gases that completely surround the earth. It extends to an altitude of 800 to 1000 kms above the earth‟s surface, but is deeper at the equator and shallow at the poles. About 99.9% of the mass occurs below 50 Km and 0.0997% between 50 and 100 km altitude. Major polluting gases/ particles are confined to the lowermost layer of atmosphere known as Troposphere that extends between 8 and 16 Kms above the earth surface. 4.2.2 The main sources of atmospheric pollution may be summarized as follows: a) The combustion of fuels to produce energy for heating and power generation both in the domestic sector as well as in the industrial sector. -

Environmental Chemistry MODULE No. 18: Composition of Atmosphere
____________________________________________________________________________________________________ Subject Chemistry Paper No and Title 4, Environmental Chemistry Module No and Title 18, Composition of Atmosphere Module Tag CHE_P4_M18 CHEMISTRY PAPER No. 4: Environmental Chemistry MODULE No. 18: Composition of atmosphere ____________________________________________________________________________________________________ TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Composition of earth’s atmosphere 3.1 Particles: formation of particulate matter, physical and chemical properties and its effects 3.2 Ions and radicals with reactions: hydroxyl and hydroperoxy in atmosphere and reaction with trace gases. 4. Summary CHEMISTRY PAPER No. 4: Environmental Chemistry MODULE No. 18: Composition of atmosphere ____________________________________________________________________________________________________ 1. Learning Outcomes-: After studying this module, you will: 1. Be able to differentiate between air and atmosphere. 2. Know about chemical composition of earth’s atmosphere. 3. Know the physical and chemical properties of particles, ions and radicals. 4. Know the effect of particles, ions, and radicals. 5. Understand the reactions of ions and radicals. 6. Know the variation in earth’s atmosphere with altitude in various levels of atmospheric layers. 2. Introduction The atmosphere of the earth is the blanket of surrounding gases maintained in its place by earth’s gravity. Atmospheres of all the planets of our solar system are different but some planets have similarities between their atmospheres. This includes the four innermost planets Mercury, Venus, Earth and Mars (the ‘terrestrial’ planets) and the four outer planets Jupiter, Saturn, Uranus and Neptune (the ‘gas giant’ planets). 3. Composition of earth’s atmosphere The earth’s atmosphere is composed of air. Nitrogen, Oxygen, and Argon, constitute the major gases of the air. This is the mixture of gases, water vapour and particles.