Comparing the Atmospheric Compositions of All Planets and Giant Moons in Solar System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Composition of Pluto's Aerosols Analogues
EPSC Abstracts Vol. 13, EPSC-DPS2019-941-1, 2019 EPSC-DPS Joint Meeting 2019 c Author(s) 2019. CC Attribution 4.0 license. Chemical composition of Pluto’s aerosols analogues Lora Jovanovic (1), Thomas Gautier (1), Nathalie Carrasco (1), Véronique Vuitton (2), Cédric Wolters (2), François-Régis Orthous-Daunay (2), Ludovic Vettier (1), Laurène Flandinet (2) ([email protected]) (1) Laboratoire Atmosphères, Milieux, Observations Spatiales (LATMOS), Guyancourt, France (2) Institut de Planétologie et d’Astrophysique de Grenoble (IPAG), Grenoble, France Abstract Table 1: Types of Pluto’s aerosols analogues produced with the PAMPRE experiment. The discovery of haze in Pluto’s atmosphere on July 14th, 2015, has raised lots of questions. To help Corresponding understand the data provided by the New Horizons Composition of the gas mixture altitude on spacecraft, Pluto’s aerosols analogues were Pluto [2] synthetized and their chemical composition was 99% N2 : 1% CH4 : 500 ppm CO 400 km determined by high-resolution mass spectrometry 95% N2 : 5% CH4 : 500 ppm CO 600 km (Orbitrap technique). 2.2. High-resolution mass spectrometry 1. Introduction (HRMS) study On July 14th, 2015, when Pluto was flown by the New Horizons spacecraft, aerosols were detected in its We analyzed the soluble fraction of Pluto’s aerosols atmosphere, mainly composed of molecular nitrogen analogues, dissolved in a 50:50 (v/v)% methanol:acetonitrile mixture. The analytical N2, methane CH4, with around 500 ppm of carbon monoxide CO [1,2,3]. These aerosols aggregate into instrument used was the LTQ Orbitrap XL several thin haze layers that extend at more than (ThermoFisher Scientific). -

Lecture 23: Jupiter Solar System Jupiter's Orbit
Lecture 23: Jupiter Solar System Jupiter’s Orbit •The semi-major axis of Jupiter’s orbit is a = 5.2 AU Jupiter Sun a •Kepler’s third law relates the semi-major axis to the orbital period 1 Jupiter’s Orbit •Kepler’s third law relates the semi-major axis a to the orbital period P 2 3 ⎛ P ⎞ = ⎛ a ⎞ ⎜ ⎟ ⎜ ⎟ ⎝ years⎠ ⎝ AU ⎠ •Solving for the period P yields 3/ 2 ⎛ P ⎞ = ⎛ a ⎞ ⎜ ⎟ ⎜ ⎟ ⎝ years ⎠ ⎝ AU ⎠ •Since a = 5.2 AU for Jupiter, we obtain P = 11.9 Earth years •Jupiter’s orbit has eccentricity e = 0.048 Jupiter’s Orbit •The distance from the Sun varies by about 10% during an orbit Dperihelion = 4.95 AU Daphelion = 5.45 AU •Like Mars, Jupiter is easiest to observe during favorable opposition •At this time, the Earth-Jupiter distance is only about 3.95 AU Earth Jupiter Jupiter Sun (perihelion) (aphelion) •Jupiter appears full during favorable opposition Bulk Properties of Jupiter •Jupiter is the largest planet in the solar system •We have for the radius and mass of Jupiter Rjupiter = 71,400 km = 11.2 Rearth 30 Mjupiter = 1.9 x 10 g = 318 Mearth •The volume of Jupiter is therefore given by 4 V = π R 3 jupiter 3 jupiter 30 3 •Hence Vjupiter = 1.5 x 10 cm 2 Bulk Properties of Jupiter •The average density of Jupiter is therefore M × 30 ρ = jupiter = 1.9 10 g jupiter × 30 3 Vjupiter 1.5 10 cm •We obtain ρ = −3 jupiter 1.24 g cm •This is much less than the average density of the Earth •Hence there is little if any iron and no dense core inside Jupiter Bulk Properties of Jupiter •The average density of the Earth is equal to −3 ρ⊕ = 6 g cm •The density -

+ New Horizons
Media Contacts NASA Headquarters Policy/Program Management Dwayne Brown New Horizons Nuclear Safety (202) 358-1726 [email protected] The Johns Hopkins University Mission Management Applied Physics Laboratory Spacecraft Operations Michael Buckley (240) 228-7536 or (443) 778-7536 [email protected] Southwest Research Institute Principal Investigator Institution Maria Martinez (210) 522-3305 [email protected] NASA Kennedy Space Center Launch Operations George Diller (321) 867-2468 [email protected] Lockheed Martin Space Systems Launch Vehicle Julie Andrews (321) 853-1567 [email protected] International Launch Services Launch Vehicle Fran Slimmer (571) 633-7462 [email protected] NEW HORIZONS Table of Contents Media Services Information ................................................................................................ 2 Quick Facts .............................................................................................................................. 3 Pluto at a Glance ...................................................................................................................... 5 Why Pluto and the Kuiper Belt? The Science of New Horizons ............................... 7 NASA’s New Frontiers Program ........................................................................................14 The Spacecraft ........................................................................................................................15 Science Payload ...............................................................................................................16 -

"Is There Life on Venus?" Pdf File
Home / Space / Specials Is there life on Venus? We call them "Martians" because, in science fiction, Mars has always been the natural home of extraterrestrials. In fact, of all the planets in the solar system, Mars is the prime candidate to host life, after Earth, of course. Indeed, Mars is on the outer edge of the habitable zone, that area around the Sun that delimits the space where the temperature is low enough to ensure that the water remains in a liquid state. And where there's water, it is well known, there's probably life. Yet it's cold on Mars today, and so far, all the probes and rovers we have sent to the surface of the red planet have found no signs of life. Planet Venus At the opposite end of the habitable belt there is Venus, similar in size and "geological" characteristics to our planet – that is, like Earth, it is a rocky planet. However, unlike Earth, Venus is probably one of the most inhospitable places in the Solar System: the temperature on the ground is about 500°C, higher than that recorded on Mercury, the planet closest to the Sun, so the Sun is not directly responsible for that infernal climate. The blame lies with the very extreme greenhouse effect on Venus. We know that the greenhouse effect is due to the presence of gases that trap solar radiation and heat the atmosphere. The main greenhouse gases are carbon dioxide (CO2), methane, water vapour and nitrogen oxides. On Earth we are rightly concerned because the amount of carbon dioxide is increasing due to human activities and with the increase in CO2, temperatures are rising. -

1 the Atmosphere of Pluto As Observed by New Horizons G
The Atmosphere of Pluto as Observed by New Horizons G. Randall Gladstone,1,2* S. Alan Stern,3 Kimberly Ennico,4 Catherine B. Olkin,3 Harold A. Weaver,5 Leslie A. Young,3 Michael E. Summers,6 Darrell F. Strobel,7 David P. Hinson,8 Joshua A. Kammer,3 Alex H. Parker,3 Andrew J. Steffl,3 Ivan R. Linscott,9 Joel Wm. Parker,3 Andrew F. Cheng,5 David C. Slater,1† Maarten H. Versteeg,1 Thomas K. Greathouse,1 Kurt D. Retherford,1,2 Henry Throop,7 Nathaniel J. Cunningham,10 William W. Woods,9 Kelsi N. Singer,3 Constantine C. C. Tsang,3 Rebecca Schindhelm,3 Carey M. Lisse,5 Michael L. Wong,11 Yuk L. Yung,11 Xun Zhu,5 Werner Curdt,12 Panayotis Lavvas,13 Eliot F. Young,3 G. Leonard Tyler,9 and the New Horizons Science Team 1Southwest Research Institute, San Antonio, TX 78238, USA 2University of Texas at San Antonio, San Antonio, TX 78249, USA 3Southwest Research Institute, Boulder, CO 80302, USA 4National Aeronautics and Space Administration, Ames Research Center, Space Science Division, Moffett Field, CA 94035, USA 5The Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA 6George Mason University, Fairfax, VA 22030, USA 7The Johns Hopkins University, Baltimore, MD 21218, USA 8Search for Extraterrestrial Intelligence Institute, Mountain View, CA 94043, USA 9Stanford University, Stanford, CA 94305, USA 10Nebraska Wesleyan University, Lincoln, NE 68504 11California Institute of Technology, Pasadena, CA 91125, USA 12Max-Planck-Institut für Sonnensystemforschung, 37191 Katlenburg-Lindau, Germany 13Groupe de Spectroscopie Moléculaire et Atmosphérique, Université Reims Champagne-Ardenne, 51687 Reims, France *To whom correspondence should be addressed. -

Atmospheric Pressure
Atmospheric pressure We all know that the atmosphere of Earth exerts a pressure on all of us. This pressure is the result of a column of air bearing down on us. However, in the seventeenth century, many scientists and philosophers believed that the air had no weight, which we already proved to be untrue in the lab (Remembered the fun you had sucking air out of the POM bottle?). Evangelista Torricelli, a student of Galileo’s, proved that air has weight using another experiment. He took a glass tube longer than 760 mm that is closed at one end and filled it completely with mercury. When he inverted the tube into a dish of mercury, some of the mercury flows out, but a column of mercury remained inside the tube. Torricelli argued that the mercury surface in the dish experiences the force of Earth’s atmosphere due to gravity, which held up the column of mercury. The force exerted by the atmosphere, which depends on the atmospheric pressure, equals the weight of mercury column in the tube. Therefore, the height of the mercury column can be used as a measure of atmospheric pressure. Although Torricelli’s explanation met with fierce opposition, it also had supporters. Blaise Pascal, for example, had one of Torricelli’s barometers carried to the top of a mountain and compared its reading there with the reading on a duplicate barometer at the base of the mountain. As the barometer was carried up, the height of the mercury column decreased, as expected, because the amount of air pressing down on the mercury in the dish decreased as the instrument was carried higher. -
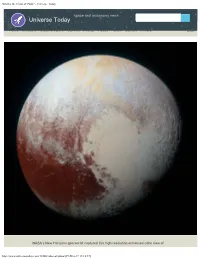
What Is the Color of Pluto? - Universe Today
What is the Color of Pluto? - Universe Today space and astronomy news Universe Today Home Members Guide to Space Carnival Photos Videos Forum Contact Privacy Login NASA’s New Horizons spacecraft captured this high-resolution enhanced color view of http://www.universetoday.com/13866/color-of-pluto/[29-Mar-17 13:18:37] What is the Color of Pluto? - Universe Today Pluto on July 14, 2015. Credit: NASA/JHUAPL/SwRI WHAT IS THE COLOR OF PLUTO? Article Updated: 28 Mar , 2017 by Matt Williams When Pluto was first discovered by Clybe Tombaugh in 1930, astronomers believed that they had found the ninth and outermost planet of the Solar System. In the decades that followed, what little we were able to learn about this distant world was the product of surveys conducted using Earth-based telescopes. Throughout this period, astronomers believed that Pluto was a dirty brown color. In recent years, thanks to improved observations and the New Horizons mission, we have finally managed to obtain a clear picture of what Pluto looks like. In addition to information about its surface features, composition and tenuous atmosphere, much has been learned about Pluto’s appearance. Because of this, we now know that the one-time “ninth planet” of the Solar System is rich and varied in color. Composition: With a mean density of 1.87 g/cm3, Pluto’s composition is differentiated between an icy mantle and a rocky core. The surface is composed of more than 98% nitrogen ice, with traces of methane and carbon monoxide. Scientists also suspect that Pluto’s internal structure is differentiated, with the rocky material having settled into a dense core surrounded by a mantle of water ice. -

Thermal Structure and Composition of Jupiter's Great Red Spot from High-Resolution Thermal Imaging
Thermal Structure and Composition of Jupiter’s Great Red Spot from High-Resolution Thermal Imaging Leigh N. Fletchera,b, G. S. Ortona, O. Mousisc,d, P. Yanamandra-Fishera, P. D. Parrishe,a, P. G. J. Irwinb, B. M. Fishera, L. Vanzif, T. Fujiyoshig, T. Fuseg, A.A. Simon-Millerh, E. Edkinsi, T.L. Haywardj, J. De Buizerk aJet Propulsion Laboratory, California Institute of Technology, 4800 Oak Grove Drive, Pasadena, CA, 91109, USA bAtmospheric, Oceanic and Planetary Physics, Department of Physics, University of Oxford, Clarendon Laboratory, Parks Road, Oxford, OX1 3PU, UK cInstitut UTINAM, CNRS-UMR 6213, Observatoire de Besan¸con, Universit´ede Franche-Comt´e, Besan¸con, France dLunar and Planetary Laboratory, University of Arizona, Tucson, AZ, USA eSchool of GeoScience, University of Edinburgh, Crew Building, King’s Buildings, Edinburgh, EH9 3JN, UK fPontificia Universidad Catolica de Chile, Department of Electrical Engineering, Av. Vicuna Makenna 4860, Santiago, Chile. gSubaru Telescope, National Astronomical Observatory of Japan, National Institutes of Natural Sciences, 650 North A’ohoku Place, Hilo, Hawaii 96720, USA hNASA/Goddard Spaceflight Center, Greenbelt, Maryland, 20771, USA iUniversity of California, Santa Barbara, Santa Barbara, CA 93106, USA jGemini Observatory, Southern Operations Center, c/o AURA, Casilla 603, La Serena, Chile. kSOFIA - USRA, NASA Ames Research Center, Moffet Field, CA 94035, USA. Abstract Thermal-IR imaging from space-borne and ground-based observatories was used to investigate the temperature, composition and aerosol structure of Jupiter’s Great Red Spot (GRS) and its temporal variability between 1995-2008. An elliptical warm core, extending over 8◦ of longitude and 3◦ of latitude, was observed within the cold anticyclonic vortex at 21◦S. -

Characterising the Three-Dimensional Ozone Distribution of a Tidally Locked Earth-Like Planet
Proedrou and Hocke Earth, Planets and Space (2016) 68:96 DOI 10.1186/s40623-016-0461-x FULL PAPER Open Access Characterising the three‑dimensional ozone distribution of a tidally locked Earth‑like planet Elisavet Proedrou1,2*† and Klemens Hocke1,2,3† Abstract We simulate the 3D ozone distribution of a tidally locked Earth-like exoplanet using the high-resolution, 3D chemistry- climate model CESM1(WACCM) and study how the ozone layer of a tidally locked Earth (TLE) (�TLE = 1/365 days) differs from that of our present-day Earth (PDE) �( PDE = 1/1 day). The middle atmosphere reaches a steady state asymptotically within the first 80 days of the simulation. An upwelling, centred on the subsolar point, is present on the day side while a downwelling, centred on the antisolar point, is present on the night side. In the mesosphere, we find similar global ozone distributions for the TLE and the PDE, with decreased ozone on the day side and enhanced ozone on the night side. In the lower mesosphere, a jet stream transitions into a large-scale vortex around a low-pressure system, located at low latitudes of the TLE night side. In the middle stratosphere, the concentration of odd oxygen is approximately equal to that of the ozone [(Ox) ≈ (O3)]. At these altitudes, the lifetime of odd oxygen is ∼16 h and the transport processes significantly contribute to the global distribution of stratospheric ozone. Compared to the PDE, where the strong Coriolis force acts as a mixing barrier between low and high latitudes, the transport processes of the TLE are governed by jet streams variable in the zonal and meridional directions. -

What Is Ozone Layer? a Layer in the Atmosphere of Earth That Protects Us from Harmful UV Rays from the Sun
International Day for Preservation of Ozone Layer 16 September 2020 Image Source: https://sfxstl.org/circleofcreation What is Ozone layer? A layer in the atmosphere of Earth that protects us from harmful UV rays from the Sun. It’s responsible for preserving life on the planet! What is happening? The ozone layer has begun to deplete because of harmful chemical substances and gases. This problem is not only contributing to global warming and climate change, but also allowing the dangerous radiation from the sun to affect human beings and ecosystems! Source: https://www.un.org/en/events/ozoneday/ What is causing its depletion? ● Human activities are the biggest cause of the ozone layer depletion ● When we burn coal, natural gas and other fuels for electricity, they release harmful gases such as carbon dioxide, nitrous oxide, etc that spread into the atmosphere and surround us like a blanket ● These harmful gases, called greenhouse gases, trap heat and radiation from the sun, which is causing the depletion as well as global warming. Clorofluorocarbons (CFCs), which are found in ACs and halogens, are other harmful greenhouse gases. Sources: https://e360.yale.edu/features/geoengineer-the-planet-more-scientists-now-say-it-must-be-an-option https://www.ucsusa.org/resources/ozone-hole-and-global-warming What can we do? Some things we can do to reduce our contribution to ozone layer depletion are: 1. Minimize use of cars. 2. Maintain your ACs regularly. 3. Avoid using products that are harmful to the environment and to us. 4. Buy local products which are more eco- friendly. -

A Study on the Concentration and Dispersion of Pm10 in Uthm by Using Simple Modelling and Meteorological Factors
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UTHM Institutional Repository i A STUDY ON THE CONCENTRATION AND DISPERSION OF PM10 IN UTHM BY USING SIMPLE MODELLING AND METEOROLOGICAL FACTORS MALEK FAIZAL B ABD RAHMAN A Project Report submitted in partial fulfilment of the requirements for the award of the Degree of Master of Engineering in Civil Engineering Faculty of Civil and Environmental Engineering Universiti Tun Hussein Onn Malaysia FEBRUARY, 2013 v ABSTRACT Air pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere. Air pollution can also be known as degradation of air quality resulting from unwanted chemicals or other materials occurring in the air. The simple way to know how polluted the air is to calculate the amounts of foreign and/or natural substances occurring in the atmosphere that may result in adverse effects to humans, animals, vegetation and/or materials. The objective of this study is to create a simulation of air quality dispersion in UTHM campus by using computer aided design mechanism such as software and calculating tools. Another objective is to compare the concentration obtained from the end result of calculation with past studies. The air pollutant in the scope of study is Particulate Matter (PM10). The highest reading recorded for E-Sampler was 305µg/m3. It was recorded in Structure Lab sampling point while the highest expected concentration by the Gaussian Dispersion Model was 184µg/m3 for UTHM Stadium. -

Mercury Friday, February 23
ASTRONOMY 161 Introduction to Solar System Astronomy Class 18 Mercury Friday, February 23 Mercury: Basic characteristics Mass = 3.302×1023 kg (0.055 Earth) Radius = 2,440 km (0.383 Earth) Density = 5,427 kg/m³ Sidereal rotation period = 58.6462 d Albedo = 0.11 (Earth = 0.39) Average distance from Sun = 0.387 A.U. Mercury: Key Concepts (1) Mercury has a 3-to-2 spin-orbit coupling (not synchronous rotation). (2) Mercury has no permanent atmosphere because it is too hot. (3) Like the Moon, Mercury has cratered highlands and smooth plains. (4) Mercury has an extremely large iron-rich core. (1) Mercury has a 3-to-2 spin-orbit coupling (not synchronous rotation). Mercury is hard to observe from the Earth (because it is so close to the Sun). Its rotation speed can be found from Doppler shift of radar signals. Mercury’s unusual orbit Orbital period = 87.969 days Rotation period = 58.646 days = (2/3) x 87.969 days Mercury is NOT in synchronous rotation (1 rotation per orbit). Instead, it has 3-to-2 spin-orbit coupling (3 rotations for 2 orbits). Synchronous rotation (WRONG!) 3-to-2 spin-orbit coupling (RIGHT!) Time between one noon and the next is 176 days. Sun is above the horizon for 88 days at the time. Daytime temperatures reach as high as: 700 Kelvin (800 degrees F). Nighttime temperatures drops as low as: 100 Kelvin (-270 degrees F). (2) Mercury has no permanent atmosphere because it is too hot (and has low escape speed). Temperature is a measure of the 3kT v = random speed of m atoms (or v typical speed of atom molecules).