The Surface Compositions of Pluto and Charon ⇑ D.P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Wunda-Full World? Carbon Dioxide Ice Deposits on Umbriel and Other Uranian Moons
Icarus 290 (2017) 1–13 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus A Wunda-full world? Carbon dioxide ice deposits on Umbriel and other Uranian moons ∗ Michael M. Sori , Jonathan Bapst, Ali M. Bramson, Shane Byrne, Margaret E. Landis Lunar and Planetary Laboratory, University of Arizona, Tucson, AZ 85721, USA a r t i c l e i n f o a b s t r a c t Article history: Carbon dioxide has been detected on the trailing hemispheres of several Uranian satellites, but the exact Received 22 June 2016 nature and distribution of the molecules remain unknown. One such satellite, Umbriel, has a prominent Revised 28 January 2017 high albedo annulus-shaped feature within the 131-km-diameter impact crater Wunda. We hypothesize Accepted 28 February 2017 that this feature is a solid deposit of CO ice. We combine thermal and ballistic transport modeling to Available online 2 March 2017 2 study the evolution of CO 2 molecules on the surface of Umbriel, a high-obliquity ( ∼98 °) body. Consid- ering processes such as sublimation and Jeans escape, we find that CO 2 ice migrates to low latitudes on geologically short (100s–1000 s of years) timescales. Crater morphology and location create a local cold trap inside Wunda, and the slopes of crater walls and a central peak explain the deposit’s annular shape. The high albedo and thermal inertia of CO 2 ice relative to regolith allows deposits 15-m-thick or greater to be stable over the age of the solar system. -

Chemical Composition of Pluto's Aerosols Analogues
EPSC Abstracts Vol. 13, EPSC-DPS2019-941-1, 2019 EPSC-DPS Joint Meeting 2019 c Author(s) 2019. CC Attribution 4.0 license. Chemical composition of Pluto’s aerosols analogues Lora Jovanovic (1), Thomas Gautier (1), Nathalie Carrasco (1), Véronique Vuitton (2), Cédric Wolters (2), François-Régis Orthous-Daunay (2), Ludovic Vettier (1), Laurène Flandinet (2) ([email protected]) (1) Laboratoire Atmosphères, Milieux, Observations Spatiales (LATMOS), Guyancourt, France (2) Institut de Planétologie et d’Astrophysique de Grenoble (IPAG), Grenoble, France Abstract Table 1: Types of Pluto’s aerosols analogues produced with the PAMPRE experiment. The discovery of haze in Pluto’s atmosphere on July 14th, 2015, has raised lots of questions. To help Corresponding understand the data provided by the New Horizons Composition of the gas mixture altitude on spacecraft, Pluto’s aerosols analogues were Pluto [2] synthetized and their chemical composition was 99% N2 : 1% CH4 : 500 ppm CO 400 km determined by high-resolution mass spectrometry 95% N2 : 5% CH4 : 500 ppm CO 600 km (Orbitrap technique). 2.2. High-resolution mass spectrometry 1. Introduction (HRMS) study On July 14th, 2015, when Pluto was flown by the New Horizons spacecraft, aerosols were detected in its We analyzed the soluble fraction of Pluto’s aerosols atmosphere, mainly composed of molecular nitrogen analogues, dissolved in a 50:50 (v/v)% methanol:acetonitrile mixture. The analytical N2, methane CH4, with around 500 ppm of carbon monoxide CO [1,2,3]. These aerosols aggregate into instrument used was the LTQ Orbitrap XL several thin haze layers that extend at more than (ThermoFisher Scientific). -

The Subsurface Habitability of Small, Icy Exomoons J
A&A 636, A50 (2020) Astronomy https://doi.org/10.1051/0004-6361/201937035 & © ESO 2020 Astrophysics The subsurface habitability of small, icy exomoons J. N. K. Y. Tjoa1,?, M. Mueller1,2,3, and F. F. S. van der Tak1,2 1 Kapteyn Astronomical Institute, University of Groningen, Landleven 12, 9747 AD Groningen, The Netherlands e-mail: [email protected] 2 SRON Netherlands Institute for Space Research, Landleven 12, 9747 AD Groningen, The Netherlands 3 Leiden Observatory, Leiden University, Niels Bohrweg 2, 2300 RA Leiden, The Netherlands Received 1 November 2019 / Accepted 8 March 2020 ABSTRACT Context. Assuming our Solar System as typical, exomoons may outnumber exoplanets. If their habitability fraction is similar, they would thus constitute the largest portion of habitable real estate in the Universe. Icy moons in our Solar System, such as Europa and Enceladus, have already been shown to possess liquid water, a prerequisite for life on Earth. Aims. We intend to investigate under what thermal and orbital circumstances small, icy moons may sustain subsurface oceans and thus be “subsurface habitable”. We pay specific attention to tidal heating, which may keep a moon liquid far beyond the conservative habitable zone. Methods. We made use of a phenomenological approach to tidal heating. We computed the orbit averaged flux from both stellar and planetary (both thermal and reflected stellar) illumination. We then calculated subsurface temperatures depending on illumination and thermal conduction to the surface through the ice shell and an insulating layer of regolith. We adopted a conduction only model, ignoring volcanism and ice shell convection as an outlet for internal heat. -

+ New Horizons
Media Contacts NASA Headquarters Policy/Program Management Dwayne Brown New Horizons Nuclear Safety (202) 358-1726 [email protected] The Johns Hopkins University Mission Management Applied Physics Laboratory Spacecraft Operations Michael Buckley (240) 228-7536 or (443) 778-7536 [email protected] Southwest Research Institute Principal Investigator Institution Maria Martinez (210) 522-3305 [email protected] NASA Kennedy Space Center Launch Operations George Diller (321) 867-2468 [email protected] Lockheed Martin Space Systems Launch Vehicle Julie Andrews (321) 853-1567 [email protected] International Launch Services Launch Vehicle Fran Slimmer (571) 633-7462 [email protected] NEW HORIZONS Table of Contents Media Services Information ................................................................................................ 2 Quick Facts .............................................................................................................................. 3 Pluto at a Glance ...................................................................................................................... 5 Why Pluto and the Kuiper Belt? The Science of New Horizons ............................... 7 NASA’s New Frontiers Program ........................................................................................14 The Spacecraft ........................................................................................................................15 Science Payload ...............................................................................................................16 -

1 the Atmosphere of Pluto As Observed by New Horizons G
The Atmosphere of Pluto as Observed by New Horizons G. Randall Gladstone,1,2* S. Alan Stern,3 Kimberly Ennico,4 Catherine B. Olkin,3 Harold A. Weaver,5 Leslie A. Young,3 Michael E. Summers,6 Darrell F. Strobel,7 David P. Hinson,8 Joshua A. Kammer,3 Alex H. Parker,3 Andrew J. Steffl,3 Ivan R. Linscott,9 Joel Wm. Parker,3 Andrew F. Cheng,5 David C. Slater,1† Maarten H. Versteeg,1 Thomas K. Greathouse,1 Kurt D. Retherford,1,2 Henry Throop,7 Nathaniel J. Cunningham,10 William W. Woods,9 Kelsi N. Singer,3 Constantine C. C. Tsang,3 Rebecca Schindhelm,3 Carey M. Lisse,5 Michael L. Wong,11 Yuk L. Yung,11 Xun Zhu,5 Werner Curdt,12 Panayotis Lavvas,13 Eliot F. Young,3 G. Leonard Tyler,9 and the New Horizons Science Team 1Southwest Research Institute, San Antonio, TX 78238, USA 2University of Texas at San Antonio, San Antonio, TX 78249, USA 3Southwest Research Institute, Boulder, CO 80302, USA 4National Aeronautics and Space Administration, Ames Research Center, Space Science Division, Moffett Field, CA 94035, USA 5The Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA 6George Mason University, Fairfax, VA 22030, USA 7The Johns Hopkins University, Baltimore, MD 21218, USA 8Search for Extraterrestrial Intelligence Institute, Mountain View, CA 94043, USA 9Stanford University, Stanford, CA 94305, USA 10Nebraska Wesleyan University, Lincoln, NE 68504 11California Institute of Technology, Pasadena, CA 91125, USA 12Max-Planck-Institut für Sonnensystemforschung, 37191 Katlenburg-Lindau, Germany 13Groupe de Spectroscopie Moléculaire et Atmosphérique, Université Reims Champagne-Ardenne, 51687 Reims, France *To whom correspondence should be addressed. -
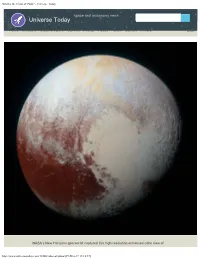
What Is the Color of Pluto? - Universe Today
What is the Color of Pluto? - Universe Today space and astronomy news Universe Today Home Members Guide to Space Carnival Photos Videos Forum Contact Privacy Login NASA’s New Horizons spacecraft captured this high-resolution enhanced color view of http://www.universetoday.com/13866/color-of-pluto/[29-Mar-17 13:18:37] What is the Color of Pluto? - Universe Today Pluto on July 14, 2015. Credit: NASA/JHUAPL/SwRI WHAT IS THE COLOR OF PLUTO? Article Updated: 28 Mar , 2017 by Matt Williams When Pluto was first discovered by Clybe Tombaugh in 1930, astronomers believed that they had found the ninth and outermost planet of the Solar System. In the decades that followed, what little we were able to learn about this distant world was the product of surveys conducted using Earth-based telescopes. Throughout this period, astronomers believed that Pluto was a dirty brown color. In recent years, thanks to improved observations and the New Horizons mission, we have finally managed to obtain a clear picture of what Pluto looks like. In addition to information about its surface features, composition and tenuous atmosphere, much has been learned about Pluto’s appearance. Because of this, we now know that the one-time “ninth planet” of the Solar System is rich and varied in color. Composition: With a mean density of 1.87 g/cm3, Pluto’s composition is differentiated between an icy mantle and a rocky core. The surface is composed of more than 98% nitrogen ice, with traces of methane and carbon monoxide. Scientists also suspect that Pluto’s internal structure is differentiated, with the rocky material having settled into a dense core surrounded by a mantle of water ice. -

Results from the New Horizons Encounter with Pluto
EPSC Abstracts Vol. 11, EPSC2017-140, 2017 European Planetary Science Congress 2017 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2017 Results from the New Horizons encounter with Pluto C. B. Olkin (1), S. A. Stern (1), J. R. Spencer (1), H. A. Weaver (2), L. A. Young (1), K. Ennico (3) and the New Horizons Team (1) Southwest Research Institute, Colorado, USA, (2) Johns Hopkins University Applied Physics Laboratory, Maryland, USA (3) NASA Ames Research Center, California, USA ([email protected]) Abstract Hydra) and the various processes that would darken those surfaces over time [5]. In July 2015, the New Horizons spacecraft flew through the Pluto system providing high spatial resolution panchromatic and color visible light imaging, near-infrared composition mapping spectroscopy, UV airglow measurements, UV solar and radio uplink occultations for atmospheric sounding, and in situ plasma and dust measurements that have transformed our understanding of Pluto and its moons [1]. Results from the science investigations focusing on geology, surface composition and atmospheric studies of Pluto and its largest satellite Charon will be presented. We also describe the New Horizons extended mission. 1. Geology and Size Highlights from the geology investigation of Pluto Figure 1: The glacial ices of Sputnik Planitia. The include the discovery of a unexpected diversity of cellular pattern is a surface expression of mobile lid geomorpholgies across the surface, the discovery of a convection. The boundaries of the cells are troughs. deep basin (informally known as Sputnik Planitia) Despite it’s size of ~900,000 km2, there are no containing glacial ices undergoing mobile-lid identified craters across Sputnik Planitia. -

Deep Space Chronicle Deep Space Chronicle: a Chronology of Deep Space and Planetary Probes, 1958–2000 | Asifa
dsc_cover (Converted)-1 8/6/02 10:33 AM Page 1 Deep Space Chronicle Deep Space Chronicle: A Chronology ofDeep Space and Planetary Probes, 1958–2000 |Asif A.Siddiqi National Aeronautics and Space Administration NASA SP-2002-4524 A Chronology of Deep Space and Planetary Probes 1958–2000 Asif A. Siddiqi NASA SP-2002-4524 Monographs in Aerospace History Number 24 dsc_cover (Converted)-1 8/6/02 10:33 AM Page 2 Cover photo: A montage of planetary images taken by Mariner 10, the Mars Global Surveyor Orbiter, Voyager 1, and Voyager 2, all managed by the Jet Propulsion Laboratory in Pasadena, California. Included (from top to bottom) are images of Mercury, Venus, Earth (and Moon), Mars, Jupiter, Saturn, Uranus, and Neptune. The inner planets (Mercury, Venus, Earth and its Moon, and Mars) and the outer planets (Jupiter, Saturn, Uranus, and Neptune) are roughly to scale to each other. NASA SP-2002-4524 Deep Space Chronicle A Chronology of Deep Space and Planetary Probes 1958–2000 ASIF A. SIDDIQI Monographs in Aerospace History Number 24 June 2002 National Aeronautics and Space Administration Office of External Relations NASA History Office Washington, DC 20546-0001 Library of Congress Cataloging-in-Publication Data Siddiqi, Asif A., 1966 Deep space chronicle: a chronology of deep space and planetary probes, 1958-2000 / by Asif A. Siddiqi. p.cm. – (Monographs in aerospace history; no. 24) (NASA SP; 2002-4524) Includes bibliographical references and index. 1. Space flight—History—20th century. I. Title. II. Series. III. NASA SP; 4524 TL 790.S53 2002 629.4’1’0904—dc21 2001044012 Table of Contents Foreword by Roger D. -

Exploring the Atmosphere of Pluto
Exploring the Atmosphere of Pluto Pluto radius… 1184 km Pluto's atmosphere consists of a thin 22 Pluto mass…. 1.3x10 kg envelope consisting of 90% nitrogen, and 10% methane, and traces of carbon Pluto temperature: Closest to sun…. 55 kelvin (-218 C) monoxide gases. These are produced by Farthest from sun… 33 kelvin (-240 C) the ices of these substances on its surface. As Pluto moves away from the Sun, its atmosphere gradually freezes out and falls to the ground. When Pluto is closer to the Sun, the temperature of Pluto's solid surface increases, causing the ices to sublimate into gas. One way to compare the atmospheres of the planets is by calculating their scale heights. The scale height is the height above the surface such that 37% of the mass of the atmosphere is below this height. Mathematically, this is equal to Pluto's atmospheric details. (Credit: NASA/JHUAPL) e-1=1/2.7 of the material. From the formula for H, you can see that as Scale-height: you make your planet more massive, the kT H = scale height ‘thickness’ of the atmosphere Mg decreases. But if you have the same planet closer to the sun where it can be hotter, -23 k = 1.38x10 J/K the thickness of the atmosphere can T = temperature in kelvin increase. g = acceleration of gravity at surface M = mass of the molecule in kg It is also the case that an atmosphere composed of low-mass molecules like For Earth’s atmosphere, T = 290 k, hydrogen (smaller M) is much thicker than -26 2 M=4.8x10 kg, g = 9.8 m/sec , the atmospheres of heavier molecules then H = 8500 meters. -

Uranian and Saturnian Satellites in Comparison
Compara've Planetology between the Uranian and Saturnian Satellite Systems - Focus on Ariel Oberon Umbriel Titania Ariel Miranda Puck Julie Cas'llo-Rogez1 and Elizabeth Turtle2 1 – JPL, California Ins'tute of Technology 2 – APL, John HopKins University 1 Objecves Revisit observa'ons of Voyager in the Uranian system in the light of Cassini-Huygens’ results – Constrain planetary subnebula, satellites, and rings system origin – Evaluate satellites’ poten'al for endogenic and geological ac'vity Uranian Satellite System • Large popula'on • System architecture almost similar to Saturn’s – “small” < 200 Km embedded in rings – “medium-sized” > 200 Km diameter – No “large” satellite – Irregular satellites • Rela'vely high albedo • CO2 ice, possibly ammonia hydrates Daphnis in Keeler gap Accre'on in Rings? Charnoz et al. (2011) Charnoz et al., Icarus, in press) Porco et al. (2007) ) 3 Ariel Titania Oberon Density(kg/m Umbriel Configuraon determined by 'dal interac'on with Saturn Configura'on determined by 'dal interac'on within the rings Distance to Planet (Rp) Configuraon determined by Titania Oberon Ariel 'dal interac'on with Saturn Umbriel Configura'on determined by 'dal interac'on within the rings Distance to Planet (Rp) Evidence for Ac'vity? “Blue” ring found in both systems Product of Enceladus’ outgassing ac'vity Associated with Mab in Uranus’ system, but source if TBD Evidence for past episode of ac'vity in Uranus’ satellite? Saturn’s and Uranus’ rings systems – both planets are scaled to the same size (Hammel 2006) Ariel • Comparatively low -

Team Persephone
AIAA TEAM SPACE TRANSPORTATION DESIGN COMPETITION TEAM PERSEPHONE Submitted By: Chelsea Dalton Ashley Miller Ryan Decker Sahil Pathan Layne Droppers Joshua Prentice Zach Harmon Andrew Townsend Nicholas Malone Nicholas Wijaya Iowa State University Department of Aerospace Engineering May 10, 2018 TEAM PERSEPHONE Page I Iowa State University: Persephone Design Team Chelsea Dalton Ryan Decker Layne Droppers Zachary Harmon Trajectory & Propulsion Communications & Power Team Lead Thermal Systems AIAA ID #908154 AIAA ID #906791 AIAA ID #532184 AIAA ID #921129 Nicholas Malone Ashley Miller Sahil Pathan Joshua Prentice Orbit Design Science Science Science AIAA ID #921128 AIAA ID #922108 AIAA ID #761247 AIAA ID #922104 Andrew Townsend Nicholas Wijaya Structures & CAD Trajectory & Propulsion AIAA ID #820259 AIAA ID #644893 TEAM PERSEPHONE Page II Contents 1 Introduction & Problem Background 2 1.1 Motivation & Background . 2 1.2 Mission Definition . 3 2 Mission Overview 5 2.1 Trade Study Tools . 5 2.2 Mission Architecture . 6 2.3 Planetary Protection . 6 3 Science 8 3.1 Observations of Interest . 8 3.2 Goals . 9 3.3 Instrumentation . 10 3.3.1 Visible and Infrared Imaging|Ralph . 11 3.3.2 Radio Science Subsystem . 12 3.3.3 Atmosphere . 14 3.3.4 Solar Wind Around Pluto . 14 3.3.5 Descent Probes . 16 4 Trajectory 19 4.1 Interplanetary Trajectory Design . 19 4.2 Earth Launch . 19 4.2.1 Launch Vehicle Selection . 19 4.2.2 Launch Vehicle Integration . 22 4.2.3 Launch Characteristics . 23 4.3 Interplanetary Cruise . 25 4.4 Jupiter Gravity Assist . 26 4.5 Pluto Orbit Insertion . 28 5 Primary Mission 30 5.1 Design Methodology . -

Journey to the Edge of the Solar System
Home / Space/ Special Reports Journey to the edge of the solar system History of a space discovery The existence of a planet beyond the orbit of Neptune had been predicted since the 1840s. Astronomers of the time, in fact, thought there was another large planet as yet unknown, situated on the edge of the Solar System, responsible for the disturbances and changes to the orbits of Uranus and Neptune. Complex mathematical calculations based on the known mass of Neptune showed, in fact, that its orbit, as well as that of nearby Uranus, did not perfectly correspond to the predictions on the motion of bodies in the Solar System. The search for the ninth planet started seriously in the twentieth century. Percival Lowell, founder astronomer (1894) and director of the Observatory in Flagstaff (Lowell Observatory), Arizona, dedicated the last eight years of his life to the search for Planet X, a phrase used to indicate a planet beyond Neptune. Lowell died in 1916, without being able to prove the existence of the missing planet. We had to wait another 14 years and more precisely 18 February 1930 to demonstrate the existence of Planet X. On that day, the twenty four year old Clyde Tombaugh was intent on observing celestial bodies with a blink comparator, an instrument that allows images of the sky obtained at different times to be compared. Clyde Tombaugh, a Kansas farmer with a great passion for astronomy, while never having carried out any formal studies on the subject, had got a job at the Lowell Observatory, thanks to drawings of Mars and Jupiter, which he had made using a telescope he had built with pieces of old farm machinery.