On the Atmospheres of Objects in the Kuiper Belt
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Composition of Pluto's Aerosols Analogues
EPSC Abstracts Vol. 13, EPSC-DPS2019-941-1, 2019 EPSC-DPS Joint Meeting 2019 c Author(s) 2019. CC Attribution 4.0 license. Chemical composition of Pluto’s aerosols analogues Lora Jovanovic (1), Thomas Gautier (1), Nathalie Carrasco (1), Véronique Vuitton (2), Cédric Wolters (2), François-Régis Orthous-Daunay (2), Ludovic Vettier (1), Laurène Flandinet (2) ([email protected]) (1) Laboratoire Atmosphères, Milieux, Observations Spatiales (LATMOS), Guyancourt, France (2) Institut de Planétologie et d’Astrophysique de Grenoble (IPAG), Grenoble, France Abstract Table 1: Types of Pluto’s aerosols analogues produced with the PAMPRE experiment. The discovery of haze in Pluto’s atmosphere on July 14th, 2015, has raised lots of questions. To help Corresponding understand the data provided by the New Horizons Composition of the gas mixture altitude on spacecraft, Pluto’s aerosols analogues were Pluto [2] synthetized and their chemical composition was 99% N2 : 1% CH4 : 500 ppm CO 400 km determined by high-resolution mass spectrometry 95% N2 : 5% CH4 : 500 ppm CO 600 km (Orbitrap technique). 2.2. High-resolution mass spectrometry 1. Introduction (HRMS) study On July 14th, 2015, when Pluto was flown by the New Horizons spacecraft, aerosols were detected in its We analyzed the soluble fraction of Pluto’s aerosols atmosphere, mainly composed of molecular nitrogen analogues, dissolved in a 50:50 (v/v)% methanol:acetonitrile mixture. The analytical N2, methane CH4, with around 500 ppm of carbon monoxide CO [1,2,3]. These aerosols aggregate into instrument used was the LTQ Orbitrap XL several thin haze layers that extend at more than (ThermoFisher Scientific). -

+ New Horizons
Media Contacts NASA Headquarters Policy/Program Management Dwayne Brown New Horizons Nuclear Safety (202) 358-1726 [email protected] The Johns Hopkins University Mission Management Applied Physics Laboratory Spacecraft Operations Michael Buckley (240) 228-7536 or (443) 778-7536 [email protected] Southwest Research Institute Principal Investigator Institution Maria Martinez (210) 522-3305 [email protected] NASA Kennedy Space Center Launch Operations George Diller (321) 867-2468 [email protected] Lockheed Martin Space Systems Launch Vehicle Julie Andrews (321) 853-1567 [email protected] International Launch Services Launch Vehicle Fran Slimmer (571) 633-7462 [email protected] NEW HORIZONS Table of Contents Media Services Information ................................................................................................ 2 Quick Facts .............................................................................................................................. 3 Pluto at a Glance ...................................................................................................................... 5 Why Pluto and the Kuiper Belt? The Science of New Horizons ............................... 7 NASA’s New Frontiers Program ........................................................................................14 The Spacecraft ........................................................................................................................15 Science Payload ...............................................................................................................16 -

1 the Atmosphere of Pluto As Observed by New Horizons G
The Atmosphere of Pluto as Observed by New Horizons G. Randall Gladstone,1,2* S. Alan Stern,3 Kimberly Ennico,4 Catherine B. Olkin,3 Harold A. Weaver,5 Leslie A. Young,3 Michael E. Summers,6 Darrell F. Strobel,7 David P. Hinson,8 Joshua A. Kammer,3 Alex H. Parker,3 Andrew J. Steffl,3 Ivan R. Linscott,9 Joel Wm. Parker,3 Andrew F. Cheng,5 David C. Slater,1† Maarten H. Versteeg,1 Thomas K. Greathouse,1 Kurt D. Retherford,1,2 Henry Throop,7 Nathaniel J. Cunningham,10 William W. Woods,9 Kelsi N. Singer,3 Constantine C. C. Tsang,3 Rebecca Schindhelm,3 Carey M. Lisse,5 Michael L. Wong,11 Yuk L. Yung,11 Xun Zhu,5 Werner Curdt,12 Panayotis Lavvas,13 Eliot F. Young,3 G. Leonard Tyler,9 and the New Horizons Science Team 1Southwest Research Institute, San Antonio, TX 78238, USA 2University of Texas at San Antonio, San Antonio, TX 78249, USA 3Southwest Research Institute, Boulder, CO 80302, USA 4National Aeronautics and Space Administration, Ames Research Center, Space Science Division, Moffett Field, CA 94035, USA 5The Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA 6George Mason University, Fairfax, VA 22030, USA 7The Johns Hopkins University, Baltimore, MD 21218, USA 8Search for Extraterrestrial Intelligence Institute, Mountain View, CA 94043, USA 9Stanford University, Stanford, CA 94305, USA 10Nebraska Wesleyan University, Lincoln, NE 68504 11California Institute of Technology, Pasadena, CA 91125, USA 12Max-Planck-Institut für Sonnensystemforschung, 37191 Katlenburg-Lindau, Germany 13Groupe de Spectroscopie Moléculaire et Atmosphérique, Université Reims Champagne-Ardenne, 51687 Reims, France *To whom correspondence should be addressed. -
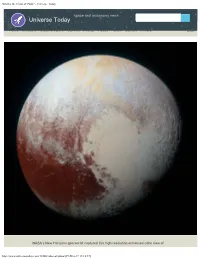
What Is the Color of Pluto? - Universe Today
What is the Color of Pluto? - Universe Today space and astronomy news Universe Today Home Members Guide to Space Carnival Photos Videos Forum Contact Privacy Login NASA’s New Horizons spacecraft captured this high-resolution enhanced color view of http://www.universetoday.com/13866/color-of-pluto/[29-Mar-17 13:18:37] What is the Color of Pluto? - Universe Today Pluto on July 14, 2015. Credit: NASA/JHUAPL/SwRI WHAT IS THE COLOR OF PLUTO? Article Updated: 28 Mar , 2017 by Matt Williams When Pluto was first discovered by Clybe Tombaugh in 1930, astronomers believed that they had found the ninth and outermost planet of the Solar System. In the decades that followed, what little we were able to learn about this distant world was the product of surveys conducted using Earth-based telescopes. Throughout this period, astronomers believed that Pluto was a dirty brown color. In recent years, thanks to improved observations and the New Horizons mission, we have finally managed to obtain a clear picture of what Pluto looks like. In addition to information about its surface features, composition and tenuous atmosphere, much has been learned about Pluto’s appearance. Because of this, we now know that the one-time “ninth planet” of the Solar System is rich and varied in color. Composition: With a mean density of 1.87 g/cm3, Pluto’s composition is differentiated between an icy mantle and a rocky core. The surface is composed of more than 98% nitrogen ice, with traces of methane and carbon monoxide. Scientists also suspect that Pluto’s internal structure is differentiated, with the rocky material having settled into a dense core surrounded by a mantle of water ice. -

Results from the New Horizons Encounter with Pluto
EPSC Abstracts Vol. 11, EPSC2017-140, 2017 European Planetary Science Congress 2017 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2017 Results from the New Horizons encounter with Pluto C. B. Olkin (1), S. A. Stern (1), J. R. Spencer (1), H. A. Weaver (2), L. A. Young (1), K. Ennico (3) and the New Horizons Team (1) Southwest Research Institute, Colorado, USA, (2) Johns Hopkins University Applied Physics Laboratory, Maryland, USA (3) NASA Ames Research Center, California, USA ([email protected]) Abstract Hydra) and the various processes that would darken those surfaces over time [5]. In July 2015, the New Horizons spacecraft flew through the Pluto system providing high spatial resolution panchromatic and color visible light imaging, near-infrared composition mapping spectroscopy, UV airglow measurements, UV solar and radio uplink occultations for atmospheric sounding, and in situ plasma and dust measurements that have transformed our understanding of Pluto and its moons [1]. Results from the science investigations focusing on geology, surface composition and atmospheric studies of Pluto and its largest satellite Charon will be presented. We also describe the New Horizons extended mission. 1. Geology and Size Highlights from the geology investigation of Pluto Figure 1: The glacial ices of Sputnik Planitia. The include the discovery of a unexpected diversity of cellular pattern is a surface expression of mobile lid geomorpholgies across the surface, the discovery of a convection. The boundaries of the cells are troughs. deep basin (informally known as Sputnik Planitia) Despite it’s size of ~900,000 km2, there are no containing glacial ices undergoing mobile-lid identified craters across Sputnik Planitia. -

The Surface Compositions of Pluto and Charon ⇑ D.P
Icarus 246 (2015) 82–92 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus The surface compositions of Pluto and Charon ⇑ D.P. Cruikshank a, , W.M. Grundy b, F.E. DeMeo c, M.W. Buie d, R.P. Binzel e, D.E. Jennings f, C.B. Olkin d, J.W. Parker d, D.C. Reuter f, J.R. Spencer d, S.A. Stern d, L.A. Young d, H.A. Weaver g a NASA Ames Research Center, Moffett Field, CA 94035, United States b Lowell Observatory, Flagstaff, AZ 86001, United States c Harvard-Smithsonian Center for Astrophysics, Cambridge, MA 02138, United States d Southwest Research Institute, Boulder, CO 80302, United States e Massachusetts Institute of Technology, Cambridge, MA 02139, United States f NASA Goddard Spaceflight Center, Greenbelt, MD 20771, United States g Applied Physics Laboratory, Johns Hopkins University, Laurel, MD 20723, United States article info abstract Article history: The surface of Pluto as it is understood on the eve of the encounter of the New Horizons spacecraft Received 6 January 2014 (mid-2015) consists of a spatially heterogeneous mix of solid N2,CH4, CO, C2H6, and an additional Revised 15 May 2014 component that imparts color, and may not be an ice. The known molecular ices are detected by Accepted 16 May 2014 near-infrared spectroscopy. The N ice occurs in the hexagonal crystalline b-phase, stable at T > 35.6 K. Available online 6 June 2014 2 Spectroscopic evidence for wavelength shifts in the CH4 bands attests to the complex mixing of CH4 and N2 in the solid state, in accordance with the phase diagram for N2 +CH4. -

Exploring the Atmosphere of Pluto
Exploring the Atmosphere of Pluto Pluto radius… 1184 km Pluto's atmosphere consists of a thin 22 Pluto mass…. 1.3x10 kg envelope consisting of 90% nitrogen, and 10% methane, and traces of carbon Pluto temperature: Closest to sun…. 55 kelvin (-218 C) monoxide gases. These are produced by Farthest from sun… 33 kelvin (-240 C) the ices of these substances on its surface. As Pluto moves away from the Sun, its atmosphere gradually freezes out and falls to the ground. When Pluto is closer to the Sun, the temperature of Pluto's solid surface increases, causing the ices to sublimate into gas. One way to compare the atmospheres of the planets is by calculating their scale heights. The scale height is the height above the surface such that 37% of the mass of the atmosphere is below this height. Mathematically, this is equal to Pluto's atmospheric details. (Credit: NASA/JHUAPL) e-1=1/2.7 of the material. From the formula for H, you can see that as Scale-height: you make your planet more massive, the kT H = scale height ‘thickness’ of the atmosphere Mg decreases. But if you have the same planet closer to the sun where it can be hotter, -23 k = 1.38x10 J/K the thickness of the atmosphere can T = temperature in kelvin increase. g = acceleration of gravity at surface M = mass of the molecule in kg It is also the case that an atmosphere composed of low-mass molecules like For Earth’s atmosphere, T = 290 k, hydrogen (smaller M) is much thicker than -26 2 M=4.8x10 kg, g = 9.8 m/sec , the atmospheres of heavier molecules then H = 8500 meters. -

Team Persephone
AIAA TEAM SPACE TRANSPORTATION DESIGN COMPETITION TEAM PERSEPHONE Submitted By: Chelsea Dalton Ashley Miller Ryan Decker Sahil Pathan Layne Droppers Joshua Prentice Zach Harmon Andrew Townsend Nicholas Malone Nicholas Wijaya Iowa State University Department of Aerospace Engineering May 10, 2018 TEAM PERSEPHONE Page I Iowa State University: Persephone Design Team Chelsea Dalton Ryan Decker Layne Droppers Zachary Harmon Trajectory & Propulsion Communications & Power Team Lead Thermal Systems AIAA ID #908154 AIAA ID #906791 AIAA ID #532184 AIAA ID #921129 Nicholas Malone Ashley Miller Sahil Pathan Joshua Prentice Orbit Design Science Science Science AIAA ID #921128 AIAA ID #922108 AIAA ID #761247 AIAA ID #922104 Andrew Townsend Nicholas Wijaya Structures & CAD Trajectory & Propulsion AIAA ID #820259 AIAA ID #644893 TEAM PERSEPHONE Page II Contents 1 Introduction & Problem Background 2 1.1 Motivation & Background . 2 1.2 Mission Definition . 3 2 Mission Overview 5 2.1 Trade Study Tools . 5 2.2 Mission Architecture . 6 2.3 Planetary Protection . 6 3 Science 8 3.1 Observations of Interest . 8 3.2 Goals . 9 3.3 Instrumentation . 10 3.3.1 Visible and Infrared Imaging|Ralph . 11 3.3.2 Radio Science Subsystem . 12 3.3.3 Atmosphere . 14 3.3.4 Solar Wind Around Pluto . 14 3.3.5 Descent Probes . 16 4 Trajectory 19 4.1 Interplanetary Trajectory Design . 19 4.2 Earth Launch . 19 4.2.1 Launch Vehicle Selection . 19 4.2.2 Launch Vehicle Integration . 22 4.2.3 Launch Characteristics . 23 4.3 Interplanetary Cruise . 25 4.4 Jupiter Gravity Assist . 26 4.5 Pluto Orbit Insertion . 28 5 Primary Mission 30 5.1 Design Methodology . -

Journey to the Edge of the Solar System
Home / Space/ Special Reports Journey to the edge of the solar system History of a space discovery The existence of a planet beyond the orbit of Neptune had been predicted since the 1840s. Astronomers of the time, in fact, thought there was another large planet as yet unknown, situated on the edge of the Solar System, responsible for the disturbances and changes to the orbits of Uranus and Neptune. Complex mathematical calculations based on the known mass of Neptune showed, in fact, that its orbit, as well as that of nearby Uranus, did not perfectly correspond to the predictions on the motion of bodies in the Solar System. The search for the ninth planet started seriously in the twentieth century. Percival Lowell, founder astronomer (1894) and director of the Observatory in Flagstaff (Lowell Observatory), Arizona, dedicated the last eight years of his life to the search for Planet X, a phrase used to indicate a planet beyond Neptune. Lowell died in 1916, without being able to prove the existence of the missing planet. We had to wait another 14 years and more precisely 18 February 1930 to demonstrate the existence of Planet X. On that day, the twenty four year old Clyde Tombaugh was intent on observing celestial bodies with a blink comparator, an instrument that allows images of the sky obtained at different times to be compared. Clyde Tombaugh, a Kansas farmer with a great passion for astronomy, while never having carried out any formal studies on the subject, had got a job at the Lowell Observatory, thanks to drawings of Mars and Jupiter, which he had made using a telescope he had built with pieces of old farm machinery. -

NASA PSD R&A Reorganization OPAG Perspective
Outer Solar System: Many Worlds to Explore Welcome to OPAG Meeting 26 Flagstaff Arizona August 11-12, 2016 Alfred McEwen University of Arizona OPAG Steering Committee • Many thanks to outgoing members: – Candy Hansen (former chair), Jani Radebaugh, Kevin Baines, Jack Connerney, Bill McKinnon (another former chair), Heidi Hammell • Welcome to new members: – Jeff Bowman, Scott Edgington, Amanda Hendrix, Terry Hurford, Carol Paty, Kunio Sayanagi • Continuing members: – Jason Barnes, Pat Beauchamp, Mark Hofstadter, Alfred McEwen (chair), Jeff Moore, Julie Rathbun, Britney Schmidt, Linda Spilker, Zibi Turtle OPAG Agenda August 11-12, 2016 August 12: August 11: 8:00 coffee, posters 8:00 coffee, registration, posters Future Missions 8:20 Welcome, meeting objectives - Alfred McEwen (University of Arizona) 8:30 Roadmaps to Ocean Worlds (ROW)– Amanda Hendrix (PSI), Terry 8:30 PSD and outer Solar System exploration updates – Curt Niebur (NASA HQ) Hurford (GSFC) 9:00 Q&A Q&A break, posters 9:15 Discussion of R&A reorganization impact on OPAG - Jared Leisner (NASA HQ) 9:45 Q&A 10:00 Vision and Voyages discussion, future Decadal Survey break recommendations - Larry Soderblom (USGS emeritus) 10:00 New Frontiers 4 draft AO - Curt Niebur (NASA HQ) Q&A Q&A 11:00 Ice giant mission study report – Mark Hofstadter (JPL) 11:30 ESA M5 Saturn Probe concept -- Olivier Mousis (Aix-Marseille Updates on Current Missions Université) 10:45 JUICE – Nicolas Altobelli (ESA, ESAC) (remote call-in) Q&A 11:15 Cassini – Linda Spilker (JPL) 11:45 Update on Space Launch System -

The Atmosphere of Pluto As Observed by New Horizons
RESEARCH ◥ has a bluish color, suggesting a composition of RESEARCHARTICLESUMMARY very small particles. The observed scattering prop- erties of the haze are consistent with a tholin-like composition. Buoyancy waves generated by winds PLANETARY SCIENCE flowing over orography can produce vertically propagating compression and rarefaction waves that may be related to the narrow haze layers. The atmosphere of Pluto as Pluto’s cold upper atmosphere means atmo- spheric escape must occur via slow thermal observed by New Horizons Jeans’ escape. The inferred escape rate of nitro- gen is ~10,000 times slower than predicted, G. Randall Gladstone,* S. Alan Stern, Kimberly Ennico, Catherine B. Olkin, whereas that of methane is about the same Harold A. Weaver, Leslie A. Young, Michael E. Summers, Darrell F. Strobel, as predicted. The low nitrogen loss rate is con- David P. Hinson, Joshua A. Kammer, Alex H. Parker, Andrew J. Steffl, Ivan R. Linscott, sistent with an undetected Charon atmosphere Joel Wm. Parker, Andrew F. Cheng, David C. Slater,† Maarten H. Versteeg, but possibly inconsistent with sublimation/ Thomas K. Greathouse, Kurt D. Retherford, Henry Throop, Nathaniel J. Cunningham, erosional features seen on Pluto’s surface, so William W. Woods, Kelsi N. Singer, Constantine C. C. Tsang, Eric Schindhelm, that past escape rates may have been much Carey M. Lisse, Michael L. Wong, Yuk L. Yung, Xun Zhu, larger at times. Capture of escaping methane Werner Curdt, Panayotis Lavvas, Eliot F. Young, G. Leonard Tyler, and photochemical products by Charon, and the New Horizons Science Team subsequent surface chemical reactions, may con- tribute to the reddish color of its north pole. -
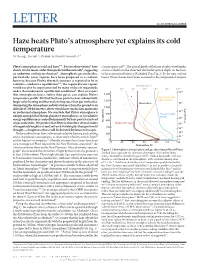
Haze Heats Pluto's Atmosphere Yet Explains Its Cold Temperature
LETTER doi:10.1038/nature24465 Haze heats Pluto’s atmosphere yet explains its cold temperature Xi Zhang1, Darrell F. Strobel2 & Hiroshi Imanaka3,4 Pluto’s atmosphere is cold and hazy1–3. Recent observations1 have Cassini spacecraft12. The optical depth of the haze at other wavelengths shown it to be much colder than predicted theoretically4, suggesting can be scaled from the observed ultraviolet optical depth on the basis an unknown cooling mechanism1. Atmospheric gas molecules, of the extinction efficiency (Extended Data Fig. 2). In the near-surface particularly water vapour, have been proposed as a coolant; layers, Pluto’s hazes have been assumed to be composed of organic however, because Pluto’s thermal structure is expected to be in 4–9 radiative–conductive equilibrium , the required water vapour Density (cm–3) would need to be supersaturated by many orders of magnitude 106 108 1010 1012 1014 1016 9 under thermodynamic equilibrium conditions . Here we report 10–8 that atmospheric hazes, rather than gases, can explain Pluto’s 1,400 temperature profile. We find that haze particles have substantially Gas-only model 1,200 without water larger solar heating and thermal cooling rates than gas molecules, Observation 10–7 dominating the atmospheric radiative balance from the ground to an 1,000 altitude of 700 kilometres, above which heat conduction maintains 10–6 an isothermal atmosphere. We conclude that Pluto’s atmosphere is 800 Pre-encounter unique among Solar System planetary atmospheres, as its radiative model 10–5 energy equilibrium is controlled primarily by haze particles instead 600 Altitude (km) Pressure (Pa) of gas molecules.