Ozone, O3, Is a Form of Oxygen; a Blue Gas with a Pungent Odor Noticeable When Gas Is Formed by an Electrical Discharge
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Appendix 1 Some Astrophysical Reminders
Appendix 1 Some Astrophysical Reminders Marc Ollivier 1.1 A Physics and Astrophysics Overview 1.1.1 Star or Planet? Roughly speaking, we can say that the physics of stars and planets is mainly governed by their mass and thus by two effects: 1. Gravitation that tends to compress the object, thus releasing gravitational energy 2. Nuclear processes that start as the core temperature of the object increases The mass is thus a good parameter for classifying the different astrophysical objects, the adapted mass unit being the solar mass (written Ma). As the mass decreases, three categories of objects can be distinguished: ∼ 1. if M>0.08 Ma ( 80MJ where MJ is the Jupiter mass) the mass is sufficient and, as a consequence, the gravitational contraction in the core of the object is strong enough to start hydrogen fusion reactions. The object is then called a “star” and its radius is proportional to its mass. 2. If 0.013 Ma <M<0.08 Ma (13 MJ <M<80 MJ), the core temperature is not high enough for hydrogen fusion reactions, but does allow deuterium fu- sion reactions. The object is called a “brown dwarf” and its radius is inversely proportional to the cube root of its mass. 3. If M<0.013 Ma (M<13 MJ) the temperature a the center of the object does not permit any nuclear fusion reactions. The object is called a “planet”. In this category one distinguishes giant gaseous and telluric planets. This latter is not massive enough to accrete gas. The mass limit between giant and telluric planets is about 10 terrestrial masses. -

"High Accuracy Rotation--Vibration Calculations on Small Molecules" In
High Accuracy Rotation–Vibration Calculations on Small Molecules Jonathan Tennyson Department of Physics and Astronomy, University College London, London, UK 1 INTRODUCTION comprehensive understanding requires the knowledge of many millions, perhaps even billions, of individual transi- High-resolution spectroscopy measures the transitions bet- tions (Tennyson et al. 2007). It is not practical to measure ween energy levels with high accuracy; typically, uncer- this much data in the laboratory and therefore a more real- tainties are in the region of 1 part in 108. Although it is istic approach is the development of an accurate theoretical possible, under favorable circumstances, to obtain this sort model, benchmarked against experiment. of accuracy by fitting effective Hamiltonians to observed The incompleteness of most experimental datasets means spectra (see Bauder 2011: Fundamentals of Rotational that calculations are useful for computing other properties Spectroscopy, this handbook), such ultrahigh accuracy is that can be associated with spectra. The partition functions largely beyond the capabilities of purely ab initio proce- and the variety of thermodynamic properties that are linked dures. Given this, it is appropriate to address the question to this (Martin et al. 1991) are notable among these. Again, of why it is useful to calculate the spectra of molecules ab calculations are particularly useful for estimating these initio (Tennyson 1992). quantities at high temperature (e.g., Neale and Tennyson The concept of the potential energy surfaces, which in 1995). turn is based on the Born–Oppenheimer approximation, Other more fundamental reasons for calculating spectra underpins nearly all of gas-phase chemical physics. The include the search for unusual features, such as clustering of original motivation for calculating spectra was to provide energy levels (Jensen 2000) or quantum monodromy (Child stringent tests of potential energy surfaces. -

Atmospheric Pressure
Atmospheric pressure We all know that the atmosphere of Earth exerts a pressure on all of us. This pressure is the result of a column of air bearing down on us. However, in the seventeenth century, many scientists and philosophers believed that the air had no weight, which we already proved to be untrue in the lab (Remembered the fun you had sucking air out of the POM bottle?). Evangelista Torricelli, a student of Galileo’s, proved that air has weight using another experiment. He took a glass tube longer than 760 mm that is closed at one end and filled it completely with mercury. When he inverted the tube into a dish of mercury, some of the mercury flows out, but a column of mercury remained inside the tube. Torricelli argued that the mercury surface in the dish experiences the force of Earth’s atmosphere due to gravity, which held up the column of mercury. The force exerted by the atmosphere, which depends on the atmospheric pressure, equals the weight of mercury column in the tube. Therefore, the height of the mercury column can be used as a measure of atmospheric pressure. Although Torricelli’s explanation met with fierce opposition, it also had supporters. Blaise Pascal, for example, had one of Torricelli’s barometers carried to the top of a mountain and compared its reading there with the reading on a duplicate barometer at the base of the mountain. As the barometer was carried up, the height of the mercury column decreased, as expected, because the amount of air pressing down on the mercury in the dish decreased as the instrument was carried higher. -

Characterising the Three-Dimensional Ozone Distribution of a Tidally Locked Earth-Like Planet
Proedrou and Hocke Earth, Planets and Space (2016) 68:96 DOI 10.1186/s40623-016-0461-x FULL PAPER Open Access Characterising the three‑dimensional ozone distribution of a tidally locked Earth‑like planet Elisavet Proedrou1,2*† and Klemens Hocke1,2,3† Abstract We simulate the 3D ozone distribution of a tidally locked Earth-like exoplanet using the high-resolution, 3D chemistry- climate model CESM1(WACCM) and study how the ozone layer of a tidally locked Earth (TLE) (�TLE = 1/365 days) differs from that of our present-day Earth (PDE) �( PDE = 1/1 day). The middle atmosphere reaches a steady state asymptotically within the first 80 days of the simulation. An upwelling, centred on the subsolar point, is present on the day side while a downwelling, centred on the antisolar point, is present on the night side. In the mesosphere, we find similar global ozone distributions for the TLE and the PDE, with decreased ozone on the day side and enhanced ozone on the night side. In the lower mesosphere, a jet stream transitions into a large-scale vortex around a low-pressure system, located at low latitudes of the TLE night side. In the middle stratosphere, the concentration of odd oxygen is approximately equal to that of the ozone [(Ox) ≈ (O3)]. At these altitudes, the lifetime of odd oxygen is ∼16 h and the transport processes significantly contribute to the global distribution of stratospheric ozone. Compared to the PDE, where the strong Coriolis force acts as a mixing barrier between low and high latitudes, the transport processes of the TLE are governed by jet streams variable in the zonal and meridional directions. -

The Rotational Spectrum of Protonated Sulfur Dioxide, HOSO+
A&A 533, L11 (2011) Astronomy DOI: 10.1051/0004-6361/201117753 & c ESO 2011 Astrophysics Letter to the Editor The rotational spectrum of protonated sulfur dioxide, HOSO+ V. Lattanzi1,2, C. A. Gottlieb1,2, P. Thaddeus1,2, S. Thorwirth3, and M. C. McCarthy1,2 1 Harvard-Smithsonian Center for Astrophysics, 60 Garden St., Cambridge, MA 02138, USA e-mail: [email protected] 2 School of Engineering & Applied Sciences, Harvard University, 29 Oxford St., Cambridge, MA 02138, USA 3 I. Physikalisches Institut, Universität zu Köln, Zülpicher Str. 77, 50937 Köln, Germany Received 21 July 2011 / Accepted 19 August 2011 ABSTRACT Aims. We report on the millimeter-wave rotational spectrum of protonated sulfur dioxide, HOSO+. Methods. Ten rotational transitions between 186 and 347 GHz have been measured to high accuracy in a negative glow discharge. Results. The present measurements improve the accuracy of the previously reported centimeter-wave spectrum by two orders of magnitude, allowing a frequency calculation of the principal transitions to about 4 km s−1 in equivalent radial velocity near 650 GHz, or one linewidth in hot cores and corinos. Conclusions. Owing to the high abundance of sulfur-bearing molecules in many galactic molecular sources, the HOSO+ ion is an excellent candidate for detection, especially in hot cores and corinos in which SO2 and several positive ions are prominent. Key words. ISM: molecules – radio lines: ISM – molecular processes – molecular data – line: identification 1. Introduction abundant in hot regions; (6) and radio observations of SH+ and SO+ (Menten et al. 2011; Turner 1994) have established that pos- Molecules with sulfur account for about 10% of the species iden- itive ions of sulfur-bearing molecules are surprisingly abundant tified in the interstellar gas and circumstellar envelopes. -

What Is Ozone Layer? a Layer in the Atmosphere of Earth That Protects Us from Harmful UV Rays from the Sun
International Day for Preservation of Ozone Layer 16 September 2020 Image Source: https://sfxstl.org/circleofcreation What is Ozone layer? A layer in the atmosphere of Earth that protects us from harmful UV rays from the Sun. It’s responsible for preserving life on the planet! What is happening? The ozone layer has begun to deplete because of harmful chemical substances and gases. This problem is not only contributing to global warming and climate change, but also allowing the dangerous radiation from the sun to affect human beings and ecosystems! Source: https://www.un.org/en/events/ozoneday/ What is causing its depletion? ● Human activities are the biggest cause of the ozone layer depletion ● When we burn coal, natural gas and other fuels for electricity, they release harmful gases such as carbon dioxide, nitrous oxide, etc that spread into the atmosphere and surround us like a blanket ● These harmful gases, called greenhouse gases, trap heat and radiation from the sun, which is causing the depletion as well as global warming. Clorofluorocarbons (CFCs), which are found in ACs and halogens, are other harmful greenhouse gases. Sources: https://e360.yale.edu/features/geoengineer-the-planet-more-scientists-now-say-it-must-be-an-option https://www.ucsusa.org/resources/ozone-hole-and-global-warming What can we do? Some things we can do to reduce our contribution to ozone layer depletion are: 1. Minimize use of cars. 2. Maintain your ACs regularly. 3. Avoid using products that are harmful to the environment and to us. 4. Buy local products which are more eco- friendly. -

A Study on the Concentration and Dispersion of Pm10 in Uthm by Using Simple Modelling and Meteorological Factors
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UTHM Institutional Repository i A STUDY ON THE CONCENTRATION AND DISPERSION OF PM10 IN UTHM BY USING SIMPLE MODELLING AND METEOROLOGICAL FACTORS MALEK FAIZAL B ABD RAHMAN A Project Report submitted in partial fulfilment of the requirements for the award of the Degree of Master of Engineering in Civil Engineering Faculty of Civil and Environmental Engineering Universiti Tun Hussein Onn Malaysia FEBRUARY, 2013 v ABSTRACT Air pollution is the introduction of chemicals, particulate matter, or biological materials that cause harm or discomfort to humans or other living organisms, or cause damage to the natural environment or built environment, into the atmosphere. Air pollution can also be known as degradation of air quality resulting from unwanted chemicals or other materials occurring in the air. The simple way to know how polluted the air is to calculate the amounts of foreign and/or natural substances occurring in the atmosphere that may result in adverse effects to humans, animals, vegetation and/or materials. The objective of this study is to create a simulation of air quality dispersion in UTHM campus by using computer aided design mechanism such as software and calculating tools. Another objective is to compare the concentration obtained from the end result of calculation with past studies. The air pollutant in the scope of study is Particulate Matter (PM10). The highest reading recorded for E-Sampler was 305µg/m3. It was recorded in Structure Lab sampling point while the highest expected concentration by the Gaussian Dispersion Model was 184µg/m3 for UTHM Stadium. -
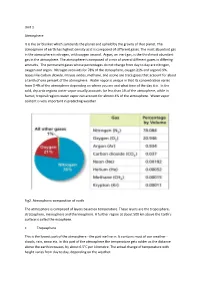
Unit 1 Atmosphere It Is the Air Blanket Which Surrounds the Planet And
Unit 1 Atmosphere It is the air blanket which surrounds the planet and upheld by the gravity of that planet. The atmosphere of earth has highest density as it is composed of different gases. The most abundant gas in the atmosphere is nitrogen, with oxygen second. Argon, an inert gas, is the third most abundant gas in the atmosphere. The atmosphere is composed of a mix of several different gases in differing amounts. The permanent gases whose percentages do not change from day to day are nitrogen, oxygen and argon. Nitrogen accounts for 78% of the atmosphere, oxygen 21% and argon 0.9%. Gases like carbon dioxide, nitrous oxides, methane, and ozone are trace gases that account for about a tenth of one percent of the atmosphere. Water vapor is unique in that its concentration varies from 0-4% of the atmosphere depending on where you are and what time of the day it is. In the cold, dry artic regions water vapor usually accounts for less than 1% of the atmosphere, while in humid, tropical regions water vapor can account for almost 4% of the atmosphere. Water vapor content is very important in predicting weather. Fig2. Atmospheric composition of earth The atmosphere is comprised of layers based on temperature. These layers are the troposphere, stratosphere, mesosphere and thermosphere. A further region at about 500 km above the Earth's surface is called the exosphere. • Troposphere This is the lowest part of the atmosphere - the part we live in. It contains most of our weather - clouds, rain, snow etc. In this part of the atmosphere the temperature gets colder as the distance above the earth increases, by about 6.5°C per kilometre. -

6Th Grade SDP Science Teachers
Curriculum Guide for 6th Grade SDP Science Teachers Please note: Pennsylvania & Next Generation Science Standards as well as Instructional Resources are found on the SDP Curriculum Engine Prepared by :Emily McGady, Science Curriculum Specialist 8/2017 1 6th Grade (Earth) Science Curriculum Term 1 (9/5-11/13/17) Topic: Landforms Duration: 9-10 Weeks Performance Objectives SWBAT: • analyze models of Earth’s various landforms (e.g., mountains, peninsulas) IOT identify and describe these landforms. • compare and contrast different bodies of water on Earth (e.g., streams, ponds, lakes, creeks) IOT categorize water systems as lentic or lotic. • compare and contrast different water systems (e.g., wetlands, oceans, rivers, watersheds) IOT describe their relationship to each other as well as to landforms. • create a stream table IOT explore relationships between systems, water, and land. • identify features of maps and diagrams IOT interpret what models represent. • describe Earth’s natural processes IOT analyze their effects on the Earth’s systems. • give examples of weathering and erosion IOT describe the impacts of weathering and erosion on landforms. • construct a scientific explanation based on evidence IOT determine how the uneven distributions of Earth’s mineral, energy, and groundwater resources are the result of past and current geoscience processes. • construct an explanation based on evidence IOT determine how geoscience processes have changed Earth’s surface at varying time and spatial scales. Define the criteria and constraints of a design problem IOT provide sufficient precision to ensure a successful solution, taking into account relevant scientific principles and potential impacts on people and the natural environment that may limit possible solutions. -

Air Pollution
Annals of Agricultural and Environmental Medicine 2019, Vol 26, No 4, 566–571 www.aaem.pl ORIGINAL ARTICLE Air pollution: how many cigarettes does each Pole ‘smoke’ every year and how does it influence health, with special respect to lung cancer? Robert Chudzik1,A-D , Paweł Rybojad1,2,C-F , Katarzyna Jarosz-Chudzik3,B-C , Marek Sawicki1,E-F , Beata Rybojad4,5,C,E-F , Lech Panasiuk6,E-F 1 Chair and Department of Thoracic Surgery, Medical University, Lublin, Poland 2 Department of Thoracic Surgery, Holy Cross Cancer Centre, Kielce, Poland 3 Independent Public Clinical Hospital No. 4, Lublin, Poland 4 Department of Emergency Medicine, Medical University, Lublin, Poland 5 Department of Anaesthesiology and Intensive Care, Children’s University Hospital, Lublin, Poland 6 Institute of Rural Health, Lublin, Poland A – Research concept and design, B – Collection and/or assembly of data, C – Data analysis and interpretation, D – Writing the article, E – Critical revision of the article, F – Final approval of article Chudzik R, Rybojad P, Jarosz-Chudzik K, Sawicki M, Rybojad B, Panasiuk L. Air pollution: how many cigarettes does each Pole ‘smoke’ every year and how does it influence health, with special respect to lung cancer? Ann Agric Environ Med. 2019; 26(4): 566–571. doi: 10.26444/aaem/109974 Abstract Introduction. Air pollution is one of the most important issues of our times. Air quality assessment is based on the measurement of the concentration of substances formed during the combustion process and micro-particles suspended in the air in the form of an aerosol. Microscopic atmospheric particulate matters (PM) 2.5 and 10 are mixtures of organic and inorganic pollutants smaller than 2.5 and 10 µm, respectively. -

Water on the Sun: the Sun Yields More Secrets to Spectroscopy
Contemporary Physics, 1998, volume 39, number 4, pages 283 ± 294 Water on the Sun: the Sun yields more secrets to spectroscopy JONATHAN TENNYSON and OLEG L. POLYANSKY Analysis of sunlight, which started the discipline of spectroscopy, has been the key to a number of major scienti® c discoveries. Sunspots, which are much cooler than most of the Sun’s surface, have particularly rich and complicated spectra which has long been thought to be due to very hot water. The challenge of analysing this spectrum has stimulated the development of new theoretical procedures based on full quantum mechanical treatments of the vibrational and rotational motion of the water molecule. The result has been the identi® cation of novel spectral features and a deeper understanding of how excited molecules such as superheated water behave. This work has applications ranging from the models of cool star atmospheres and rocket exhausts to the possible automated detection of forest ® res. Perhaps the most interesting result is the insight given to understanding how our own atmosphere absorbs sunlight, and the possible consequences that this may have for modelling the greenhouse eŒect. 1. Spectroscopy and the Sun Sun’s photosphere. The remaining absorptions are caused In 1814 Fraunhofer allowed a beam of sunlight from a by molecules such as water and oxygen in the Earth’s narrow opening in his shutters to pass through a prism and atmosphere which also absorb sunlight. Such spectral to be projected onto a white wall. What Fraunhofer saw analysis of light, possible both at visible and non-visible was not only the colours of the rainbow, which had been wavelengths, yields the vast majority of our knowledge not observed in this manner since Newton, but also `almost only about the rest of the Universe but also about the countless strong and weak vertical lines’ [1], see ® gure 1. -

A Diatomic Molecule Is One Atom Too Few the Successful Laser Cooling of a Triatomic Molecule Paves the Way Towards the Study of Ultracold Polyatomic Molecules
VIEWPOINT A Diatomic Molecule is One Atom too Few The successful laser cooling of a triatomic molecule paves the way towards the study of ultracold polyatomic molecules. by Paul Hamilton∗ and Eric R. Hudsony hysicists considering a foray into the study of molecules are often warned that “a diatomic molecule is one atom too many!” [1]. Now John Doyle and colleagues [2] at Harvard University have Pthrown this caution to the wind and tackled laser cooling of a triatomic molecule with success, opening the door to the study of ultracold polyatomic molecules. The technique of laser cooling [3], which uses the scat- tering of laser photons and the concomitant momentum transfer to bring atoms to a near halt, has revolutionized atomic, molecular, and optical (AMO) physics. Laser cooling and an important variant known as Sisyphus cooling [4] un- derpin three Nobel prizes in physics—for magneto-optical trapping (1997), Bose-Einstein condensation (2001), and the manipulation of individual quantum systems (2012)—and are crucial to a host of quantum-assisted technologies and fundamental physics measurements. Since photons carry very little momentum and therefore reduce an atom’s velocity by just a small amount, a pre- requisite for effective laser cooling is the ability to scatter thousands of photons. Thus laser cooling has predominantly been used only to cool simple atoms, whose electronic struc- ture dictates that after a photon is absorbed, spontaneous Figure 1: Doyle and colleagues [2] have cooled SrOH molecules emission places the atomic electron back into its original using Sisyphus cooling. In this type of cooling, the SrOH state, allowing the process to repeat nearly ad infinitum.