Subtype-Specific Gout Susceptibility Loci and Enrichment of Selection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title The Exponent of Breath: The Role of Foreign Evangelical Organizations in Combating Japan's Tuberculosis Epidemic of the Early 20th Century Permalink https://escholarship.org/uc/item/32d241sf Author Perelman, Elisheva Avital Publication Date 2011 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California The Exponent of Breath: The Role of Foreign Evangelical Organizations in Combating Japan’s Tuberculosis Epidemic of the Early 20th Century By Elisheva Avital Perelman A dissertation submitted in partial satisfaction of the Requirements for the degree of Doctor of Philosophy in History in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Andrew E. Barshay, Chair Professor John Lesch Professor Alan Tansman Fall 2011 © Copyright by Elisheva Avital Perelman 2011 All Rights Reserved Abstract The Role of Foreign Evangelical Organizations in Combating Japan’s Tuberculosis Epidemic of the Early 20th Century By Elisheva Avital Perelman Doctor of Philosophy in History University of California, Berkeley Professor Andrew E. Barshay, Chair Tuberculosis existed in Japan long before the arrival of the first medical missionaries, and it would survive them all. Still, the epidemic during the period from 1890 until the 1920s proved salient because of the questions it answered. This dissertation analyzes how, through the actions of the government, scientists, foreign evangelical leaders, and the tubercular themselves, a nation defined itself and its obligations to its subjects, and how foreign evangelical organizations, including the Young Men’s Christian Association (the Y.M.C.A.) and The Salvation Army, sought to utilize, as much as to assist, those in their care. -

Proteomics of Serum Extracellular Vesicles Identifies a Novel COPD Biomarker, Fibulin-3 from Elastic Fibres
Title: Proteomics of serum extracellular vesicles identifies a novel COPD biomarker, fibulin-3 from elastic fibres Authors: Taro Koba, Yoshito Takeda*, Ryohei Narumi, Takashi Shiromizu, Yosui Nojima, Mari Ito, Muneyoshi Kuroyama, Yu Futami, Takayuki Takimoto, Takanori Matsuki, Ryuya Edahiro, Satoshi Nojima, Yoshitomo Hayama, Kiyoharu Fukushima, Haruhiko Hirata, Shohei Koyama, Kota Iwahori, Izumi Nagatomo, Mayumi Suzuki, Yuya Shirai, Teruaki Murakami, Kaori Nakanishi, Takeshi Nakatani, Yasuhiko Suga, Kotaro Miyake, Takayuki Shiroyama, Shohei Koyama, Hiroshi Kida, Takako Sasaki, Koji Ueda, Kenji Mizuguchi, Jun Adachi, Takeshi Tomonaga, Atsushi Kumanogoh Data Supplement Sample collection and approval All serum samples were collected in Osaka University Hospital and stored at -80ºC until analysis. Approval was obtained from the Osaka University Graduate School of Medicine Institutional Review Board; all patients gave written informed consent to participate in the study, and they were arrowed to fast on free will. All methods were performed in accordance with relevant guidelines and regulations. Mouse experiments were approved by the Osaka University’s Animal Care and Use Committee, and all of the animal procedures were performed following the Osaka University guidelines on animal care. Statistical analyses Statistical analyses were conducted using JMP Pro v. 14.3.0 (SAS Institute, Cary, NC, USA). Pearson’s chi-square test or Welch’s t-test was used to compare healthy controls with COPD patients. Correlations between two parameters were calculated using Spearman’s rank correlation coefficients. Differences were considered statistically significant at p < 0.05. Ward’s hierarchical cluster analysis was used to divide COPD patients into two groups by up- and down-regulated proteins. Receiver operating characteristic curves were constructed using the SRM (selected reaction monitoring) results. -
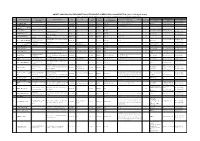
As of 15Th.April.2020) *Items Listed May Be Modified Along with the Circumstances of Supervising Organizations
ADDITIONAL ENGLISH INFORMATION OF EXCELLENT SUPERVISING ORGANIZATION (As of 15th.April.2020) *Items listed may be modified along with the circumstances of supervising organizations. In that case, we will modify the list shortly. Job Categories (Eligible when Interns shifts to No. of Person in charge Name of Organization Date of Approval Date of Expiry Accepting Country No. Name of Organization Address in English TEL HP Technical Intern Training (ii)) *Refer to Implementing TEL (in English) (Y/M/D) (Y/M/D) *Refer to Country Code Name Email Category Code Organizations* FAX ISS HOKKAIDO business 〒067-0056, 2F No.225 Mihara, Ebetsu-shi, TEL: 011-807-0491 1 ISS北海道事業協同組合 0118070491 N/A 2018/10/31 2023/10/30 CHN, VNM 1-1, 1-2, 3-21, 4-2, 4-9, 4-10, 7-6 70 佐藤(Sato) [email protected] cooperative association Hokkaido FAX: 011-807-0492 2 網走国際交流協同組合 0152462447 2018/12/10 2023/12/9 CHN 1-2, 4-2, 4-4, 4-6 7 アパレルファッション研究協同組合 3 0139551525 2018/1/22 2023/1/21 KHM 5-6 4 (事業休止中) 〒058-0204, 182-2 Erimocho Honchou, TEL: 01466-2-2211 4 えりも漁業協同組合 Erimo Fishery Cooperative 0146622211 http://www.jf-erimo.or.jp 2018/9/28 2023/9/27 IDN, VNM 2-1 5 岡道(Okamichi)(日本国内) [email protected] Horoizumi-gun, Hokkaido FAX: 01466-2-4090 〒049-2222, 2-188-1 Sawara, Mori-machi, [email protected] TEL: 01374-8-5111 5 渡島国際交流事業協同組合 N/A 0137485111 N/A 2017/11/1 2022/10/31 CHN, VNM 4-3, 4-4 18 谷垣 (TANIGAKI) Kayabe-gun, Hokkaido .jp FAX: 01374-8-5112 Okhotsk International Business 〒094-0002, 1-2-42 Masago-cho, Monbetsu-shi, 6 オホーツク国際事業協同組合 0158236686 N/A 2018/6/29 2023/6/28 CHN, THA 4-3, 4-4, 4-5 18 Association Hokkaido OKHOTSK INTERNATIONAL 〒098-1702, 91-5 Oumu, Oumu-cho, Monbetsu- [email protected]. -

2018 JSAE Annual Congress (Spring)
22001188 JSAJSAEE AnAnnnualual CongressCongress ((SpringSpring)) Wednesday, May 23 - Friday, May 25 2018 / Pacifico Yokohama Final Program Floor Map Customer Service EV EV EV EV EV Inter Continental Yokohama Grand Annex Exhibition Hall Hall May 23, 24メインホール Forum May 24 Keynote Address Conference Center セルフ On-site Registration ビジネスセンター EV EV Beverages Book Store Print Service EV EV EV EV EV Workshop 2F Harbor Lounge (Please proceed from the Exhibition Hall) Help Desk ① ② Forum ③ ④ Registration ⑤ ⑥ Registration Automotive Engineering Exposition 2018 ⑦ ⑧ Pre-registered Attendee ⑨ ⑩ ⑪ ⑫ Lecture/ Special Lecture EV EV Room 419 Tea Room Room Room 315 418 Presentation Room Presentation Room Room Presentation Room Room 417 314 + + 416 313 Room May 24 Awards Ceremony Room 312 415 + JSAE Annual Meeting + 311 414 Room 512 トイレ使用不可 Beverages + Room 511 502 Room May 24 JSAE Annual Room 503 Room Room Party 413 Room 302 304 501 Room Room Room 412 Room Registration 411 Chairperson 301 303 YOKOHAMA Booth No.98 NAGOYA Booth No.186 Speaker Room Room Room 316 317 318 Cloak Room Presentation Preparation Room Smoking Area Beverages Smoking Area 春季大会プログラム英文..indd 1 18/04/04 17:01 2018 JSAE Annual Congress (Spring) Period : Wednesday, May 23 to Friday, May 25, 2018 Venue : PACIFICO YOKOHAMA Table of Contents Timetable Wednesday, May 23 …………………………………………… 2,3 Thursday, May 24 ………………………………………………… 4,5 Friday, May 25 …………………………………………………… 6,7 Information …………………………………………………………………… 8,9 Events………………………………………………………………………… 10,11 Forum ………………………………………………………………………… 12-20 -

Subtype-Specific Gout Susceptibility Loci and Enrichment of Selection
Ann Rheum Dis: first published as 10.1136/annrheumdis-2019-216644 on 1 April 2020. Downloaded from Crystal arthropathies EPIDEMIOLOGICAL SCIENCE Subtype- specific gout susceptibility loci and enrichment of selection pressure on ABCG2 and ALDH2 identified by subtype genome- wide meta- analyses of clinically defined gout patients Akiyoshi Nakayama,1,2 Masahiro Nakatochi ,3 Yusuke Kawamura,1,4 Ken Yamamoto,5 Hirofumi Nakaoka,6 Seiko Shimizu,1 Toshihide Higashino,1,7 Teruhide Koyama,8 Asahi Hishida,9 Kiyonori Kuriki,10 Miki Watanabe,11 Toru Shimizu,12,13 Keiko Ooyama,14 Hiroshi Ooyama,14 Mitsuo Nagase,15 Yuji Hidaka,16 Daisuke Matsui,8 Takashi Tamura,9 Takeshi Nishiyama,11 Chisato Shimanoe,17,18 Sakurako Katsuura- Kamano,19 Naoyuki Takashima,20,21 Yuya Shirai,22,23 Makoto Kawaguchi,1,24 Mikiya Takao,1,25 Ryo Sugiyama,1 Yuzo Takada,26 Takahiro Nakamura,27 Hiroshi Nakashima,28 Masashi Tsunoda,28 Inaho Danjoh,29 Atsushi Hozawa,30 Kazuyoshi Hosomichi,31 Yu Toyoda,32 Yu Kubota,32 Tappei Takada,32 Hiroshi Suzuki,32 Blanka Stiburkova,33,34 Tanya J. Major,35 Tony R. Merriman,35 Nagato Kuriyama,8 Haruo Mikami,36 Toshiro Takezaki,37 Keitaro Matsuo,38,39 Sadao Suzuki,11 Tatsuo Hosoya,40,41 Yoichiro Kamatani,42,43 Michiaki Kubo,44 Kimiyoshi Ichida,40,45 Kenji Wakai,9 Ituro Inoue,6 Yukinori Okada ,22,46 Nariyoshi Shinomiya,1 Hirotaka Matsuo,1 on behalf of Japan Gout Genomics Consortium (Japan Gout) copyright. Handling editor Josef S Smolen ABSTRact Key messages ► Additional material is Objectives Genome- wide meta- analyses of clinically published online only. To view defined gout were performed to identify subtype- please visit the journal online What is already known about this subject? (http:// dx. -

15Th World Conference on Earthquake Engineering 2012 (15WCEE)
15th World Conference on Earthquake Engineering 2012 (15WCEE) Lisbon, Portugal 24-28 September 2012 Volume 1 of 38 ISBN: 978-1-63439-651-6 Printed from e-media with permission by: Curran Associates, Inc. 57 Morehouse Lane Red Hook, NY 12571 Some format issues inherent in the e-media version may also appear in this print version. Copyright© (2012) by Sociedade Portuguesa de Engenharia Sismica (SPES) All rights reserved. Printed by Curran Associates, Inc. (2015) For permission requests, please contact Sociedade Portuguesa de Engenharia Sismica (SPES) at the address below. Sociedade Portuguesa de Engenharia Sismica (SPES) Avenida do Brazil, 101 1700-066 Lisboa, Portugal Phone: +351.218443833 Fax: +351.218443035 [email protected] Additional copies of this publication are available from: Curran Associates, Inc. 57 Morehouse Lane Red Hook, NY 12571 USA Phone: 845-758-0400 Fax: 845-758-2634 Email: [email protected] Web: www.proceedings.com TABLE OF CONTENTS VOLUME 1 How Effective Are EC8 and Recommended AASHTO-LRFD Criteria for Regular Seismic Behavior of Ductile Bridges with Unequal Height Piers? ................................................................................................................................................................1 Joseph Guirguis, Sameh Mehanny Shake Table Acceleration Tracking Performance Impact on Dynamic Similitude Preliminary Findings ............................................11 Mike Mota, Franklin Moon, Ahmet Emin Aktan Proposing the Optimized Combination of Different Isolation Bearings Subjected -

The Role of Foreign Evangelical Organizations in Combating Japan’S Tuberculosis Epidemic of the Early 20Th Century
The Exponent of Breath: The Role of Foreign Evangelical Organizations in Combating Japan’s Tuberculosis Epidemic of the Early 20th Century By Elisheva Avital Perelman A dissertation submitted in partial satisfaction of the Requirements for the degree of Doctor of Philosophy in History in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Andrew E. Barshay, Chair Professor John Lesch Professor Alan Tansman Fall 2011 © Copyright by Elisheva Avital Perelman 2011 All Rights Reserved Abstract The Role of Foreign Evangelical Organizations in Combating Japan’s Tuberculosis Epidemic of the Early 20th Century By Elisheva Avital Perelman Doctor of Philosophy in History University of California, Berkeley Professor Andrew E. Barshay, Chair Tuberculosis existed in Japan long before the arrival of the first medical missionaries, and it would survive them all. Still, the epidemic during the period from 1890 until the 1920s proved salient because of the questions it answered. This dissertation analyzes how, through the actions of the government, scientists, foreign evangelical leaders, and the tubercular themselves, a nation defined itself and its obligations to its subjects, and how foreign evangelical organizations, including the Young Men’s Christian Association (the Y.M.C.A.) and The Salvation Army, sought to utilize, as much as to assist, those in their care. With both Japanese government officials and foreign evangelical leaders employing moral entrepreneurism in their approach to the victims of the nation’s epidemic, the tubercular became pawns in the relationships between the Meiji and Taishō governments, the Y.M.C.A., The Salvation Army, St. Luke’s Hospital, and the Omi Mission. -

COVID-19 and Rheumatology
Annals of the Rheumatic Diseases publishes original work on all aspects of rheumatology ARDThe EULAR Journal Editor Josef S Smolen (Austria) and disorders of connective tissue. Laboratory Associate Editors Francis Berenbaum (France) and clinical studies are equally welcome Dimitrios Boumpas (Greece) Gerd Burmester (Germany) Editorial Board Mary Crow (USA) Daniel Aletaha (Austria) Rik Lories (Belgium) Kimme Hyrich (UK) Johan Askling (Sweden) Ingrid Lundberg (Sweden) Contact Details Rik Lories (Belgium) Sang-Cheol Bae (Korea) Gary MacFarlane (UK) Iain McInnes (UK) Xenofon Baraliakos (Germany) Xavier Mariette (France) Editorial Office Thomas Pap (Germany) Anne Barton (UK) Alberto Martini (Italy) Annals of the Rheumatic Diseases David Pisetsky (USA) Maarten Boers (The Netherlands) Marco Mattuci Cerinic (Italy) BMJ Journals, BMA House, Tavistock Square Désirée van der Heijde Maxine Breban (France) Dennis McGonagle (UK) London WCIH 9JR,UK (The Netherlands) Matthew Brown (Australia) Fred Miller (USA) Kazuhiko Yamamoto (Japan) Maya Buch (UK) Peter Nash (Australia) E: [email protected] Loreto Carmona (Spain) Michael Nurmohamed (The Methodological and Statistical Carlo Chizzolini (Switzerland) Netherlands) Production Editor Advisor Bernard Combe (France) Caroline Ospelt (Switzerland) Teresa Jobson Philip Conaghan (UK) Stian Lydersen (Norway) Monika Østensen (Norway) E: [email protected] Maurizio Cutolo (Italy) Constatino Pitzalis (UK) Social Media Advisors Nicola Dalbeth (Australia) Alessia Alunno (Italy) Jane Salmon (USA) EULAR Christian Dejaco -

All-Time Great Remy Bonjasky Makes Successful Return to the Ring, Defeats Anderson ‘Braddock’ Silva in Hard- Hitting Heavyweight Main Event
ALL-TIME GREAT REMY BONJASKY MAKES SUCCESSFUL RETURN TO THE RING, DEFEATS ANDERSON ‘BRADDOCK’ SILVA IN HARD- HITTING HEAVYWEIGHT MAIN EVENT BRUSSELS, Belgium (Oct. 6, 2012) – Three years away from the ring. Three years in which Remy Bonjasky had grown older while the new generation of prospects ripened and matured into the contenders of today. The question hanging over this fight was whether Bonjasky can hang with the prime young men of the heavyweight division – and the answer was “Yes.” Bonjasky hadn’t been offered an easy comeback either. GLORY had welcomed him with open arms but only if he was willing to test himself against a rising star and prove that he could be competitive in what is by far the kickboxing world’s toughest and most stacked division. And so Bonjasky found himself opposite Anderson ‘Braddock’ Silva, the man who nearly beat the very dangerous Badr Hari earlier this year, at Vorst Nationaal Arena. Braddock’s name may not be as big as Bonjasky’s but, make no mistake, he is not someone who puts a lot of store in fame. A win over Bonjasky would have been enormous for his career and so he put his all into the bout. The pace was incredible from opening seconds, with both men hammering low kicks into each other repeatedly as they sought to slow the opposing man down. There were extended leg-kick trades several times as the pair went back and forth, one for one, until one of them did something to break the cycle. But by the mid-point of round two it was plain to see that they were each feeling the hurt from the strikes they had taken on the thighs, strikes which would have rendered a normal man unable to walk. -

Program May 14 (Thursday) Presidential Lecturepresidential
Plenary Program May 14 (Thursday) Presidential Lecture 16:20-17:05 Plenary Lecture 01 Room: National Convention Hall Chairperson: Teruo Kawada (Kyoto University, Japan) PL01 Leptin and the Regulation of Food Intake and Body Weight Jeffrey M. Friedman Rockefeller University, USA Educational 17:05-17:50 Plenary Lecture 02 Room: National Convention Hall Chairperson: Tohru Fushiki (Ryukoku University, Japan) PL02 Functional Food Science in Japan: Present State and Perspectives Keiko Abe Symposium The University of Tokyo, Japan, the Kanagawa Academy of Science & Technology (KAST), Japan May 15 (Friday) 9:00-9:45 Plenary Lecture 03 Room: Main Hall at Conference Center (Satellite Viewing is available in Room 301-304) Sponsored Symposium Chairperson: Chizuru Nishida (World Health Organization (WHO), Switzerland) PL03 The Present Role of Industrial Food Processing in Food Systems and Its Implications for Controlling the Obesity Pandemic Carlos A. Monteiro University of São Paulo, Brazil May 16 (Saturday) Luncheon 9:00-9:45 Plenary Lecture 04 Room: Main Hall at Conference Center (Satellite Viewing is available in Room 301-304) Chairperson: Pek-Yee Chow (Federation of Asian Nutrition Societies, Singapore Nutrition and Dietetics Association (SNDA), Singapore) PL04 Multi-Stakeholders and Multi-Strategic Approaches for Food and Nutrition Security Evening Kraisid Tontisirin Mahidol University, Thailand May 17 (Sunday) FANS Report FANS 9:00-9:45 Plenary Lecture 05 Room: Main Hall at Conference Center (Satellite Viewing is available in Room 301-304) Chairperson: -

Sedimentary and Foraminiferal Evidence of the 2011 Tōhoku-Oki Tsunami on the Sendai Coastal Plain, Japan
Sedimentary Geology 282 (2012) 78–89 Contents lists available at SciVerse ScienceDirect Sedimentary Geology journal homepage: www.elsevier.com/locate/sedgeo Sedimentary and foraminiferal evidence of the 2011 Tōhoku-oki tsunami on the Sendai coastal plain, Japan Jessica E. Pilarczyk a,b,⁎, Benjamin P. Horton a, Robert C. Witter c, Christopher H. Vane d, Catherine Chagué-Goff b,e, James Goff b a Sea Level Research, Department of Earth and Environmental Science, University of Pennsylvania, 240 S. 33rd Street, Philadelphia, PA 19104‐6316, USA b Australia-Pacific Tsunami Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney 2052, Australia c United States Geological Survey/Alaska Science Center, Anchorage, AK 99508, USA d British Geological Survey, Keyworth, Nottingham NG92BY, UK e Australian Nuclear Science and Technology Organisation, Locked Bag 2001, Kirrawee DC, NSW 2232, Australia article info abstract Article history: The 2011 Tōhoku-oki megathrust earthquake (Mw 9.0) generated a tsunami that reached the Sendai coastal Received 16 March 2012 plain with flow heights of ~2 to 11 m above TP (Tokyo Peil). We examined the tsunami deposit exposed in 14 Received in revised form 23 August 2012 shallow trenches along a ~4.5‐km transect perpendicular to the coast. We primarily document the strati- Accepted 27 August 2012 graphical, sedimentological, foraminiferal and geochemical characteristics of the Tōhoku-oki tsunami deposit Available online 7 September 2012 and perform a preliminary comparison with sediments deposited by the Jōgan tsunami of A.D. 869. In the coastal forest and rice fields inundated by the Tōhoku-oki tsunami, a poorly sorted, dark brown soil is Keywords: Sendai Plain buried by a poorly sorted, brown, medium-grained sand deposit. -

Wound, Pressure Ulcer and Burn Guidelines
doi: 10.1111/1346-8138.14587 Journal of Dermatology 2018; : 1–50 GUIDELINE Wound, pressure ulcer and burn guidelines – 2: Guidelines for the diagnosis and treatment of pressure ulcers, second edition Hiroshi FUJIWARA, Zenzo ISOGAI, Ryokichi IRISAWA, Masaki OTSUKA, Takafumi KADONO, Monji KOGA, Kuninori HIROSAKI, Jun ASAI, Yoshihide ASANO, Masatoshi ABE, Masahiro AMANO, Ryuta IKEGAMI, Takayuki ISHII, Taiki ISEI, Takaaki ITO, Yuji INOUE, Yohei IWATA, Yoichi OMOTO, Hiroshi KATO, Sakae KANEKO, Hiroyuki KANOH, Tamihiro KAWAKAMI, Masakazu KAWAGUCHI, Ryuichi KUKINO, Takeshi KONO, Masanari KODERA, Keisuke SAKAI, Eiichi SAKURAI, Yasuko SARAYAMA, Yoichi SHINTANI, Miki TANIOKA, Hideaki TANIZAKI, Jun TSUJITA, Naotaka DOI, Takeshi NAKANISHI, Akira HASHIMOTO, Minoru HASEGAWA, Masahiro HAYASHI, Hideki FUJITA, Manabu FUJIMOTO, Takeo MAEKAWA, Koma MATSUO, Naoki MADOKORO, Sei-ichiro MOTEGI, Hiroshi YATSUSHIRO, Osamu YAMASAKI, Yuichiro YOSHINO, Andres Le PAVOUX, Takao TACHIBANA, Hironobu IHN BACKGROUND OF THE DRAFTING OF THE POSITION OF THE GUIDELINES FOR THE GUIDELINES FOR THE DIAGNOSIS AND DIAGNOSIS AND TREATMENT OF PRESSURE TREATMENT OF PRESSURE ULCERS ULCERS The Wound, Pressure Ulcer and Burn Guidelines Drafting Com- Guidelines are documents systematically prepared to support mittee (Table 1) was composed of members delegated by the medical experts and patients for making appropriate judg- Board of Directors of the Japanese Dermatological Associa- ments in particular clinical situations. The Japanese Society tion. The committee meetings were held gathering or through of Pressure Ulcers (JSPU) published the Guidelines for the email since October 2008, and has drafted the guidelines for Prevention and Management of Pressure Ulcers in February wounds in general, and other five related guidelines, including 2009, which has undergone revisions leading to the publish- this guideline, by taking into consideration the opinions of the ing of the fourth edition in 2015.