An Analysis of Bivalve Larval Shell Pigments Using Microraman
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Archaeomalacological Data and Paleoenvironmental
ARCHAEOMALACOLOGICAL DATA AND PALEOENVIRONMENTAL RECONSTRUCTION AT THE JUPITER INLET I SITE (8PB34A), SOUTHEAST FLORIDA by Jennifer Green A Thesis Submitted to the Faculty of The Dorothy F. Schmidt College of Arts and Letters In Partial Fulfillment of the Requirements for the Degree of Master of Arts Florida Atlantic University Boca Raton, FL May 2016 Copyright 2016 by Jennifer Green ii ACKNOWLEDGEMENTS This thesis has been a journey of growth and exploration. There are several people who deserve recognition for my path of success. First, and foremost, I could have never gotten to where I am without the love and never-ending support of my parents. My dad initially stimulated my interest in archaeology as a kid. He took me to see my first site on Captiva Island during a vacation, and since then I was hooked. In addition, my mom has always been there to listen, and provide compassion and support in any way needed. My thesis advisor Dr. Arlene Fradkin deserves special recognition. If not for her agreeing to take me on as a Master’s student, I may have never gotten the opportunity to grow as a scholar. Her meticulous zooarchaeological practices have imprinted on me and have provided the baseline for all of my future endeavors. The time she has spent side- by-side with me proof-reading all of my documents has granted me several awards and grants throughout my studies, for which I am extremely thankful. Dr. Clifford Brown is one of the most brilliant men I know and his comprehensive knowledge of archaeological science will always amaze me. -

To Down Load Appendix 1
APPENDIX 1 Chapter 1 Pictures of dominant species ................................................................................................................................. 2 Species inventory of micro-invertebrate species found ....................................................................................... 16 Bathymetry Map of Pleasant Bay ........................................................................................................................ 18 Eelgrass Locations in Pleasant Bay ..................................................................................................................... 19 Sidescan Map of Pleasant Bay ............................................................................................................................. 20 Chapter 2 Species inventory of macro-invertebrate and fish species by gear type .............................................................. 21 Chapter 3 Prey otoliths and hard parts recovered during seal scat processing ..................................................................... 24 1 Pictures of dominant species Disclaimer: biological samples were treated with ethanol and Rose Bengal in the laboratory to preserve the samples. Rose Bengal is a stain commonly used in microscopy and stains cell tissue a bright pink. This is useful in the visual detection of microscopic animals in sediment samples. An overwhelming majority of micro invertebrate species do not have common names. The common names used here are listed in Pollock’s “A Practical Guide -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Benthic Macroinvertebrate Sampling
Benthic Macroinvertebrate Sampling Norton Basin, Little Bay, Grass Hassock Channel, and the Raunt Submitted to: The Port Authority of New York and New Jersey New York State Department of Environmental Conservation Submitted by: Barry A. Vittor & Associates, Inc. Kingston, NY February 2003 TABLE OF CONTENTS 1.0 INTRODUCTION...............................................................................................1 2.0 STUDY AREA......................................................................................................3 2.1 Norton Basin........................................................................................................ 3 2.2 Little Bay ............................................................................................................. 3 2.3 Reference Areas.................................................................................................... 3 2.3.1 The Raunt .................................................................................................... 3 2.3.2 Grass Hassock Channel ............................................................................... 4 3.0 METHODS..........................................................................................................4 3.1 Benthic Grab Sampling......................................................................................... 4 4.0 RESULTS.............................................................................................................7 4.1 Benthic Macroinvertebrates................................................................................ -

Investigating Population Dynamics of Red Knot Migration Along the Georgia Coast Through Mark/Recapture Analysis of Resights: 2015 Spring Season
Investigating Population Dynamics of Red Knot Migration along the Georgia Coast through Mark/Recapture Analysis of Resights: 2015 Spring Season A Cooperative Project by: US Fish and Wildlife Service, US Geological Survey, Georgia Department of Natural Resources Non-game Program, and the Center for Conservation Biology at the College of William & Mary and Virginia Commonwealth University. Investigating Population Dynamics of Red Knot Migration along the Georgia Coast through Mark/Recapture Analysis of Resights: 2015 Spring Season Fletcher M. Smith1 Bryan D. Watts, PhD1 Jim Lyons, PhD2 Tim Keyes3 1Center for Conservation Biology at the College of William & Mary and Virginia Commonwealth University, Williamsburg, Virginia 23187. 2USGS Patuxent Wildlife Research Center, Laurel, MD. 3Georgia Department of Natural Resources, Wildlife Resources Division, Non-game Program Brunswick, Georgia 31520 Project Partners: US Fish and Wildlife Service US Geological Survey Georgia Department of Natural Resources Non-game Program The Center for Conservation Biology Recommended Citation: F.M. Smith, B.D. Watts, J. E. Lyons, and T. S. Keyes. 2016. Investigating Population Dynamics of Red Knot Migration along the Georgia Coast through Mark/Recapture Analysis of Resights: Spring 2015 Season. Center for Conservation Biology Technical Report Series, CCBTR-16-05. College of William and Mary/Virginia Commonwealth University, Williamsburg, VA. 13 pp. The Center for Conservation Biology is an organization dedicated to discovering innovative solutions to environmental problems that are both scientifically sound and practical within today’s social context. Our philosophy has been to use a general systems approach to locate critical information needs and to plot a deliberate course of action to reach what we believe are essential information endpoints. -

Discocilia and Paddle Cilia in the Larvae of Mulinia Lateralis and Spisula Solidissima (Mollusca: Bivalvia)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by College of William & Mary: W&M Publish W&M ScholarWorks VIMS Articles 12-1988 Discocilia and Paddle Cilia in the Larvae of Mulinia lateralis and Spisula solidissima (Mollusca: Bivalvia) Bernardita Campos Roger L. Mann Virginia Institute of Marine Science, [email protected] Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation Campos, Bernardita and Mann, Roger L., "Discocilia and Paddle Cilia in the Larvae of Mulinia lateralis and Spisula solidissima (Mollusca: Bivalvia)" (1988). VIMS Articles. 1693. https://scholarworks.wm.edu/vimsarticles/1693 This Article is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Reference: Biol. Bull. 175: 343—348.(December, 1988) Discocilia and Paddle Cilia in the Larvae of Mulinia lateralis and Spisula solidissima (Mollusca: Bivalvia) BERNARDITA CAMPOS' AND ROGER MANN2@* ‘¿InstituteofOceanology, University ofValparaiso, P.O. Box 13-D, Vina delMar, Chile, 2Virginia Institute ofMarine Science, School ofMarine Science, The College of William and Mary, Gloucester Point, Virginia 23062 Abstract. The bivalve larval velum contains four bands ing, oval, ciliated velum. The velum contains four bands of cilia: inner and outer preoral bands, an adoral band, of cilia: inner and outer preoral bands, an adorn! band, and a postoral band. The preoral bands of compound and a postoral band (Elston, 1980; Waller, 198 1). -

Biological Resources
Biological Evaluation Form Main CSJ: 0902-90-077 Form Prepared By: Civil Associates, Inc. Date of Evaluation: April 10, 2018 Project has no Federal nexus. Proposed Letting Date: March 2019 Project not assigned to TxDOT under the NEPA Assignment MOU District(s): Fort Worth County(ies): Tarrant Roadway Name: Dallas Road TOD Corridor/Cotton Belt Extension Limits From: Intersection of W. Dallas Road and William D. Tate Avenue Limits To: Existing Links Trail at Texan Trail Roadway Project Description: Please see the following document that has been uploaded into TXECOS: Project Description (0902-90-077).pdf The environmental review, consultation, and other actions required by applicable Federal environmental laws for this project are being, or have been, carried-out by TxDOT pursuant to 23 U.S.C. 327 and a Memorandum of Understanding dated December 16, 2014, and executed by FHWA and TxDOT. Endangered Species Act (ESA) Yes Is the action area of the proposed project within the range of federally protected species? Yes Did the USFWS IPaC system identify any endangered species that may occur or could potentially be affected by the proposed project activities? Date that the IPaC system was accessed: March 14, 2018 No Is the action area of the proposed project in suitable habitat of federally protected species? *Explain: The habitat preferences for federally listed threatened/endangered species, a brief discussion of habitat availability within the project's construction footprint, and an assessment of potential adverse effects on these federally-listed -
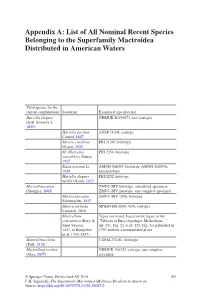
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Invertebrate Identification Guide for Chesmmap and NEAMAP Diet Analysis Studies
W&M ScholarWorks Reports 11-13-2013 Invertebrate Identification Guide for ChesMMAP and NEAMAP Diet Analysis Studies Chesapeake Bay Multispecies Monitoring and Assessment Program Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons Recommended Citation Chesapeake Bay Multispecies Monitoring and Assessment Program. (2013) Invertebrate Identification Guide for ChesMMAP and NEAMAP Diet Analysis Studies. Virginia Institute of Marine Science, William & Mary. https://doi.org/10.25773/b0y5-k411 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. 11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html -

Transportation and Dispersal of Biogenic Material in the Nearshore Marine Environment
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1974 Transportation and Dispersal of Biogenic Material in the Nearshore Marine Environment. Macomb Trezevant Jervey Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Jervey, Macomb Trezevant, "Transportation and Dispersal of Biogenic Material in the Nearshore Marine Environment." (1974). LSU Historical Dissertations and Theses. 2674. https://digitalcommons.lsu.edu/gradschool_disstheses/2674 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Checklist of Species Within the CCBNEP Study Area: References, Habitats, Distribution, and Abundance
Current Status and Historical Trends of the Estuarine Living Resources within the Corpus Christi Bay National Estuary Program Study Area Volume 4 of 4 Checklist of Species Within the CCBNEP Study Area: References, Habitats, Distribution, and Abundance Corpus Christi Bay National Estuary Program CCBNEP-06D • January 1996 This project has been funded in part by the United States Environmental Protection Agency under assistance agreement #CE-9963-01-2 to the Texas Natural Resource Conservation Commission. The contents of this document do not necessarily represent the views of the United States Environmental Protection Agency or the Texas Natural Resource Conservation Commission, nor do the contents of this document necessarily constitute the views or policy of the Corpus Christi Bay National Estuary Program Management Conference or its members. The information presented is intended to provide background information, including the professional opinion of the authors, for the Management Conference deliberations while drafting official policy in the Comprehensive Conservation and Management Plan (CCMP). The mention of trade names or commercial products does not in any way constitute an endorsement or recommendation for use. Volume 4 Checklist of Species within Corpus Christi Bay National Estuary Program Study Area: References, Habitats, Distribution, and Abundance John W. Tunnell, Jr. and Sandra A. Alvarado, Editors Center for Coastal Studies Texas A&M University - Corpus Christi 6300 Ocean Dr. Corpus Christi, Texas 78412 Current Status and Historical Trends of Estuarine Living Resources of the Corpus Christi Bay National Estuary Program Study Area January 1996 Policy Committee Commissioner John Baker Ms. Jane Saginaw Policy Committee Chair Policy Committee Vice-Chair Texas Natural Resource Regional Administrator, EPA Region 6 Conservation Commission Mr. -

Dietary Composition of Black Drum Pogonias Cromis
Dietary composition of black drum Pogonias cromis in a hypersaline estuary reflects water quality and prey availability K.S. RUBIO1, M. AJEMIAN2, G.W. STUNZ3, T.A. PALMER1, B.LEBRETON4 AND J. BESERES POLLACK1 1 Department of Life Sciences, Texas A&M University – Corpus Christi, 6300 Ocean Drive, Corpus Christi, TX 78412-5800, U.S.A. 2 Florida Atlantic University, Harbor Branch Oceanographic Institute, Fort Pierce, FL, 34946, U.S.A. 3 Harte Research Institute for Gulf of Mexico Studies, Texas A&M University—Corpus Christi, 6300 Ocean Drive, SL2 101 Corpus Christi, TX 78412-5869, U.S.A. 4 UMR Littoral, Environment et Societies, CNRS – University of La Rochelle, Institut du littoral et de l’environnement, 2 rue Olympe de Gouges, 17000 La Rochelle, France Correspondence J. Beseres Pollack Department of Life Sciences, Texas A&M University – Corpus Christi, 6300 Ocean Drive, Corpus Christi, TX 78412-5800, U.S.A. Email: [email protected] Funding information Accepted Article This article has been accepted for publication in the Journal of Fish Biology and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/jfb.13654 This article is protected by copyright. All rights reserved. The authors thank the organizations and agencies that helped fund this research, including the Texas Coastal Bend Bays & Estuaries Program (CBBEP), Harvey Weil Sportsman Conservation Award, Texas Sea Grant, and Texas A&M University-Corpus Christi. Running headline: POGONIAS CROMIS DIET IN A HYPERSALINE ESTUARY The Baffin Bay estuary is a hypersaline system in the Gulf of Mexico that supports an important recreational and commercial fishery for black drum Pogonias cromis, a benthic predator.