Buying Time: a User's Manual Foreword
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

YLO89 Magazine
•YLO 91_Layout 1 2/13/13 3:02 PM Page 1 TRD COVER Youth Leaders Only / Music Resource Book / Volume 91 / Spring 2013 Cover: Red 25 Ways To Create A Crisis Page 6 When Volunteers Date Kids Page 8 The Crisis Head/Heart Disconnect Page 10 INSIDE: ConGRADulations! Class of 2013 Music-Media Grad Gift Page 18 Heart of the Artist: RED Page 15 Jeremy Camp Page 16 The American Bible Challenge: Jeff Foxworthy Interview Page 12 Worship Chord Charts from Gungor, Planetshakers, Elevation Worship, Everfound Page 42 y r t s i & n i c i M s u h t M u o g Y n i n z i i a m i i x d a e M M CRISIS: ® HANDLING THOSE “UH OH!” SITUATIONS •YLO 91_Layout 1 2/13/13 3:02 PM Page 2 >> TRD TABLE OF CONTENTS CONTENTS MAIN/MILD/HOT ARE LISTED ALPHABETICALLY BY ARTIST 6 8 10 11 FEATURE 25 Ways To Create When Volunteers The Crisis I’m In A ARTICLES: Your Own Crisis Date Kids Disconnect Crisis NOW MAIN: 18 20 25 CONGRADULATIONS! PURPOSE FILM Artist: CLASS OF 2013 DVD GUNGOR Album Title: ConGRADulations! Class of 2013 Purpose A Creation Liturgy (Live) Song Title: Unstoppable Beautiful Things Study Theme: Sacrifice Life; Purpose Meaning Renewal MILD: 21 22 23 ELEVATION Artist: CAPITAL KINGS WORSHIP EVERFOUND Album Title: Capital Kings Nothing Is Wasted Everfound Song Title: You’ll Never Be Alone Nothing Is Wasted Never Beyond Repair Study Theme: God’s Presence Difficulty; Hope Within Grace HOT: 24 26 28 Artist: FLYLEAF JEKOB JSON Album Title: New Horizons Faith Hope Love Growing Pains Song Title: New Horizons Love Is All Brand New Study Theme: Hope; In God Love; Unconditional -

View This Page
14 發光的城市 A R O U N D T O W N FRIDAY, JANUARY 29, 2010 • TAIPEI TIMES BY AndreW C.C. HuanG MUSIC STOP South Korean superstar rains supreme COMPILED BY Ian BarthOLOmeW ubbed the Usher or Entertainment (his former Justin Timberlake of agent) because he did not have rivate information leaked TV idol Peter Ho (何潤東), Chinese Asia because of his double eyelids, a traditional onto the Internet has provided interests have approached Hsu charismatic presence standard of beauty in Asia, ample material for Taiwan’s and her leading man as product and hip-hop-driven he refused to resort to plastic Pgossip rags, but the most recent spokespersons for a range of music, South Korean surgery as many South Korean scandal surrounding the posting wedding apparel. According to superstar Rain entertainers have done, and of transsexual TV host Li Ching’s Next Magazine, the deal is worth (Jeong Ji-hoon) is a force to through sheer will and talent (利菁) medical history on a public NT$10 million each. be reckoned with. As an all- hit the big time, redefining Web site has hit all kinds of Hsu has also hit the headlines D round entertainer, excelling in audiences’ aesthetic tastes in nerves in the entertainment and for a series of new pro-vegetarian singing, dancing and acting, he the process. media industries. ads for People for the Ethical sets a new high for superstars MBLAQ, the boy group The entertainer, whose real Treatment of Animals (PETA) in Asia. launched by Rain’s own name is Regine Wu Ming-enn Asia. -
1200 Micrograms 1200 Micrograms 2002 Ibiza Heroes of the Imagination 2003 Active Magic Numbers 2007 the Time Machine 2004
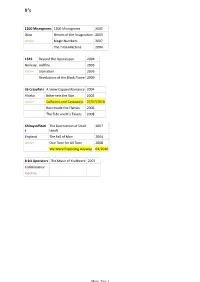
#'s 1200 Micrograms 1200 Micrograms 2002 Ibiza Heroes of the Imagination 2003 Active Magic Numbers 2007 The Time Machine 2004 1349 Beyond the Apocalypse 2004 Norway Hellfire 2005 Active Liberation 2003 Revelations of the Black Flame 2009 36 Crazyfists A Snow Capped Romance 2004 Alaska Bitterness the Star 2002 Active Collisions and Castaways 27/07/2010 Rest Inside the Flames 2006 The Tide and It's Takers 2008 65DaysofStati The Destruction of Small 2007 c Ideals England The Fall of Man 2004 Active One Time for All Time 2008 We Were Exploding Anyway 04/2010 8-bit Operators The Music of Kraftwerk 2007 Collaborative Inactive Music Page 1 A A Forest of Stars The Corpse of Rebirth 2008 United Kingdom Opportunistic Thieves of Spring 2010 Active A Life Once Lost A Great Artist 2003 U.S.A Hunter 2005 Active Iron Gag 2007 Open Your Mouth For the Speechless...In Case of Those 2000 Appointed to Die A Perfect Circle eMOTIVe 2004 U.S.A Mer De Noms 2000 Active Thirteenth Step 2003 Abigail Williams In the Absence of Light 28/09/2010 U.S.A In the Shadow of A Thousand Suns 2008 Active Abigor Channeling the Quintessence of Satan 1999 Austria Fractal Possession 2007 Active Nachthymnen (From the Twilight Kingdom) 1995 Opus IV 1996 Satanized 2001 Supreme Immortal Art 1998 Time Is the Sulphur in the Veins of the Saint... Jan 2010 Verwüstung/Invoke the Dark Age 1994 Aborted The Archaic Abattoir 2005 Belgium Engineering the Dead 2001 Active Goremageddon 2003 The Purity of Perversion 1999 Slaughter & Apparatus: A Methodical Overture 2007 Strychnine.213 2008 Aborym -
A Commentary on the Sceptical Chymist of Robert Boyle Vol. 1 of 2
A Commentary on The Sceptical Chymist of Robert Boyle Vol. 1 of 2 by Conleth Patrick Loonan, M.Sc., M.Litt. Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy to Maynooth University Presented to: The Department of Philosophy 31st October, 2014 Supervisor and Head of Department: Dr Michael Dunne TABLE OF CONTENTS TO VOL. 1 Quotation Acknowledgements Abstract Page no. Introduction: i Explanatory Notes ii Introduction to Commentary iii Methodology xi General Considerations xi The Rationale Underlying Commentary xi Choice of Edition xi Style of Commentary xi Practical Considerations xii The Process of Commenting xiii Literature Review 1 Introduction 2 Part 1: 2 Works on the History of Chemistry and of Alchemy 3 Henry M. Leicester, The Historical Background of Chemistry 3 E.J. Holmyard, Alchemy 4 James R. Partington, A History of Chemistry, vol.2 5 Maurice P. Crosland, Historical Studies in the Language of Chemistry 5 Robert P. Multhauf, The Origins of Chemistry 6 William H. Brock, The Norton History of Chemistry 6 William R. Newman, Promethean Ambitions. Alchemy and the Quest to Perfect Nature 7 TABLE OF CONTENTS TO VOL. 1 Works on the Scientific Revolution 7 E.J. Dijksterhuis, The Mechanization of the World Picture 7 A. Rupert Hall, From Galileo to Newton 9 A Work Treating Specifically of Boyle 11 Marie Boas, Robert Boyle and Seventeenth-Century Chemistry 11 Part 2: 11 M. M. Pattison Muir, (Introduction to) The Sceptical Chymist 12 E. A. Moelwyn-Hughes, (Introduction to) The Sceptical Chymist 17 Tenney L. Davis, ‘The First Edition of The Sceptical Chymist’ 19 Louis Trenchard More, The Life and Works of the Honourable Robert Boyle 21 Marie Boas, ‘An Early Version of Boyle’s: Sceptical Chymist’ 27 Jan V. -

Postcolonial and Hyperreal Translations of Australian Poetry
At the Limits: Postcolonial and Hyperreal Translations of Australian Poetry By Bridie McCarthy (BA Hons) Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Deakin University August, 2006 DEAKIN UNIVERSITY CANDIDATE DECLARATION I certify that the thesis entitled At the Limits: Postcolonial & Hyperreal Translations of Australian Poetry submitted for the degree of Doctor of Philosophy is the result of my own work and that where reference is made to the work of others, due acknowledgment is given. I also certify that any material in the thesis which has been accepted for a degree or diploma by any university or institution is identified in the text. Full Name.......................Bridie McCarthy..........................……….………. (Please Print) Signed ..................................................................................………………… Date......................................................................................…………………. AT THE LIMITS Contents Abstract i Acknowledgements iii Translator’s Note v Epigraph vii Introduction: (Un)mapping the Poetics of Postcoloniality & Hyperreality. Foreword: The Limits of Australia as Text. ix i. The Limits of Postcolonial Studies xiv i.i Historical Materialism versus Discursive Deconstruction xv i.ii Postcolonial Futures & the “Ethics of Becoming”. xviii ii. The Limits of Hyperreal Studies xx ii.i The Hyperreal at the Limits of the “Real” xxi ii.ii Baudrillardian Discourse as a Limit to Postcolonial Studies xxiv iii. Los limites de los estudios latinoamericanos xxv [The Limits of Latin American Studies] iii.i América Latina como frontera al poscolonialismo xxvii [Latin America as Border to Postcolonialism] iii.ii Del discurso latinoamericano xxx [On Latin American Discourse] iv. At the Limits of Transnationalism xxxi Afterword: Theoretical Interrogations xxxv Part One: Australia: The Postcolonial at the Limits of the Hyperreal. -

Dead by Sunrise out of Ashes Full Album Zip
Dead By Sunrise, Out Of Ashes Full Album Zip Dead By Sunrise, Out Of Ashes Full Album Zip 1 / 3 2 / 3 Dead By Sunrise - Out Of Ashes [2009].zip. -1; Size 82 MB. Fast download for credit 1 second – 0,01 €. Slow download for free 8 minut – 0 €. -1; Copy in .... Out of Ashes is the only studio album by American rock band Dead By Sunrise. Released on October 13,2009 Genre:Alternative Metal .... Dead by Sunrise - Out of Ashes LP Vinyl NM Chester Bennington Linkin Park. ... Vinyl CLASSIC ROCK Record Albums MOST 1ST PRESSING Beatles ELP .... Hi wazzup fellas, this is the '08 album of Dead By Sunrise (OUT OF ASHES). DBS is the project band of Chester Bennington of Linkin Park.. Jump to Out of Ashes (2005–2009) — Dead by Sunrise's debut studio album, Out of Ashes, was released worldwide on October 13, 2009. Contents. 1 History.. Artist: Dead by Sunrise. Title: Out of Ashes, Modified Item: No. Record Label: Warner Bros. Custom Bundle: No. Non-Domestic Product: No, Style: Alternative/ .... Kelly Finnigan - The Tales People Tell (2019) Full Album mp3 m4a iTunes rar Free ... Download Creed - Greatest Hits rar zip 320kbps [iTunes Plus AAC M4A] ... Download Dead By Sunrise - Out Of Ashes (2009) ITunes Rip M4A NimitMak .... Out of Ashes is the only studio album by American rock band Dead by Sunrise, which consisted of Linkin Park lead vocalist Chester Bennington and Amir .... 320 kbit/s Album in .rar oder .zip Archiv- Download-Links von Mega.co.nz. 8 Oct 2018 . Title: Dead By Sunrise, Out Of Ashes Full Album 45, Author: . -

Primal Fear - the Mattson 2 - Hammerfall - Dinosaur Jr
Ano XVIII - 2009 * NÚMERO 104 ISSN 1415 - 2460 - 1415 ISSN MECHANICS Caminhando reto por caminhos tortos Edguy - Girlschool - Mechanics - Primal Fear - The Mattson 2 - Hammerfall - Dinosaur Jr. e mais: Rock no Cinema - - Kurt Cobain - Loki, o documentário sobre Arnaldo Baptista Edição 105 - Junho-Julho/2009 1 14 Edguy 26 Primal Fear 03 Editorial 17 Girlschool 30 The Mattson 2 + Índice + Expediente 04 News 21 Mechanics 33 Hammerfall 08 Eventos 36 Ears Up 42 Play 43 Jukebox 44 Cinema Edição 105 - Junho-Julho/2009 2 EDITORIAL Dynamite # 105 * Junho/Julho/2009 Publisher e jornalista responsável: a edição passada André “Pomba” Cagni – Mtb: 34553 ([email protected]) prometemos Editor: Bruno Palma Fernandes ([email protected]) N uma mudança Diagramação: Rodrigo “Khall” Ramos ([email protected]) de visual e eis aqui a Foto da capa: Divulgação nova versão da revista Marketing: Hanilton Scofield ([email protected]) Dynamite. Por algum Inti Anny Queiroz ([email protected]) tempo a disponibilizamos Webmaster: Daniel Zsigmond ([email protected]) Administrativo: Alexandre Carvalho ([email protected]) na internet do jeitinho Relações Públicas: Lílian Vituzzo ([email protected]) que a publicávamos no papel. Agora os tempos Colaboraram nesta edição: Bruno Palma Fernandes, Cal Crepaldi, Carlos Eduardo são outros e por isso Couto, Dum de Lucca Neto, Everton “Pardal” Soares, Felipe Teixeira, Fernando adaptamos o formato Carpaneda, Guilherme Sorgine, Herik Correia Rocha, Humberto Finatti, Maíra Hirose, especialmente para a tela Marcos Hermes, Otávio Silvares, Ricardo Rizzo, Rodrigo “Khall” Ramos, Saulo Wanderley, do computador, tornando a leitura mais fácil e dinâmica. Thanira Rates, Vicky Machado, Zanone Fraissat. -

Populární Hudba Zahraniční
Číslo Rok Autor Název Styl 3151 rok:2005 …And You Will Know Us by the Trail of Dead Worlds apart alterantive rock 5645 rok:2006 …And You Will Know Us by the Trail of Dead So divided alternative rock 3979 rok:2000 10CC /populární kapela ze 70.let/ Best of the 70's soft rock 4816 rok:1995 2 Unlimited /nejv ětší hity legendární disco formace/ Hits Unlimited disco/dance 4177 rok:1996 2Pac /fantastické dvojalbum zast řelené hip-hop hv ězdy/ All eyez on me - 2CD hip-hop 5696 rok:2005 3 Doors Down Seventeen days post-grunge 2008 rok:2002 3 Doors Down /pro fandy:Creed, Nickelback/ Away from the sun post-grunge 5483 rok:2002 30 Seconds to Mars 30 Seconds to Mars alter..rock/EMO 5637 rok:2009 30 Seconds to Mars This is war Emo/alter-prog r. 3706 rok:2007 30 Seconds to Mars /pro fandy:My Chemical Romance/ A Beautiful EMO/alter.rock 6432 rok:2013 30 Seconds to Mars Love lust faith & dreams symph/alter./rock 5676 rok:2006 36 Crazyfists Rest inside the flames EMO/hard-core 2329 rok:2004 36 Crazyfists /zní to jako:Lostprophets,SystemOfADown/ A snow capped romance EMO/nu metal 1169 rok:2001 4 Hero Creating patterns nu-jazz 3127 rok:1992 4 Non Blondes /jedna super deska s hitem What's up/ Bigger, better, faster, more! rock 5858 rok:2009 50 Cent Before i self destruct + DVD hip-hop 3162 rok:2005 50 Cent The Massacre hip-hop 4130 rok:2007 50 Cent /nové album/ Curtis hip-hop 6485 rok:2012 69 Eyes X gothic rock/metal 3930 rok:2002 69 Eyes /pro fandy:HIM,Paradise Lost/ Paris kills gothic metal 2162 rok:2000 69 Eyes /zní to jako: HIM,Type O Negative,Sisters -

National News Obituary
JULY 22, 2017 CURRENT AFFAIRS Telangana government puts cap on weight of school bags The Telangana government has imposed a cap on the weight of bags carried by schoolchildren in order to prevent any resulting physical effect and anxiety disorder in students. ● The government issued an official order on 18 July 2017 detailing the allowed weight limit of school bags for students studying in the primary section to those in Class X. NATIONAL ● The order has been issued to all the school managements. NEWS ● The maximum weight of a school bag with textbooks and notebooks NATIONAL should not exceed 1.5 kg for children in Class 1 and II and it should NEWS not go above 2-3 kg for children studying in Class III, IV and V. ● The weight limit for students studying in Classes VI and VII is 4 kg, while for those in Classes VIII and IX it is 4.5 kg. Chester Bennington, lead singer of Linkin Park, dies Chester Bennington, the lead singer of Linkin Park, passed away on 20 July 2017 in California, U.S. He was 41. ● Bennington apparently hanged himself, which lead to his death. OBITUARY ● Born on 20 March 1976, Chester Bennington was an American singer and songwriter. ● He was best known as the frontman for the rock band Linkin Park. NATIONAL ● He was also the lead singer for Dead by Sunrise and fronted Stone NEWS Temple Pilots from 2013 to 2015. ● He first gained prominence as a vocalist following the release of Linkin Park's debut album, Hybrid Theory, in 2000. -

Challenge the Good News Paper
TM THE GOOD NEWS PAPER No. 415 SSEEINGEEING TTHEHE LLIGHTIGHT AAMIDMID SSUFFERINGUFFERING Losing a child rocks your world, your marriage and your belief in God to its core, as well-known Christian singer songwriter Steven Curtis Chapman can testify, but he determined it would only make him stronger. teven says that as a child he “grew up in a was the reality of having a relationship with Jesus relatively happy home”, although his parents that meant the most to me. ‘This is a good day,’ I fought often and his father “carried a heavy thought. Sload of shame and anger” that he sometimes “What God did in my heart that day in a small took out on Steven and his older brother Herbie. Kentucky church when I was just an eight year The atmosphere at home changed profoundly, old little boy was eternally signifi cant in my life.” however, when Steven was seven years old and his SUCCESSFUL CAREER parents and brother committed their lives to Christ The other defi ning infl uence in Steven’s life was during a gospel revival at their church. music. Steven’s dad ran a music store and taught Steven, himself, didn’t respond at the time but the guitar, so even before he was eight, Steven was later that year, seeing the change in his parents, he reading music and song writing. was challenged by the Scripture where Jesus says, He and Herbie would perform together as the “Behold, I stand at the door and knock. If anyone Chapman Brothers – he on guitar and Herbie will open the door, I will come in.” singing. -

Chester Bennington's Sidoprojekt Dead by Sunrise Släpper Debutalbumet "Out of Ashes"
2009-08-04 08:03 CEST Chester Bennington's sidoprojekt Dead By Sunrise släpper debutalbumet "Out Of Ashes". Chester Bennington är i vanliga fall sångare i ett av världens främsta rockband, Linkin Park. Nu släpper han debutalbumet "Out Of Ashes" med sitt andra band "Dead By Sunrise". Albumet släpps till hösten via Warner Bros. Records. "Ouf Of Ashes" består av Ryan Shuck (gitarr), Amir Derakh (bas), Brandon Belsky (trummor) och Chester Bennington (sång). Albumet "Out Of Ashes" spelades in i Los Angeles, Howard Benson har producerat. Han har tidigare jobbat med band som Motorhead och My Chemical Romance. Låtarna på "Out Of Ashes" började ta form när Linkin Park hade en ledig period innan de började spela in dubbelplatina albumet "Minutes To Midnight". Chester berättar själv att han skrev en del låtar som kändes och lät riktigt bra, men det var inte i linje med hur Linkin Park låter. "They were darker and moodier than anything I'd come up with for the band. So I decided to work on them on my own rather than turn them over and have them transformed into Linkin Park tracks." Bennington kallar Dead By Sunrise's låtar för mörkare, sexigare och mer personlig än någonting han skrivit förr. Hip-hop referenserna är borta och Bennington's röst får en ny framtoning på "Out Of Ashes". Bennington var även själv involverad i hela inspelningsprocessen och är mycket nöjd med resultatet Albumet "Out Of Ashes" släpps i höst! http://vids.myspace.com/index.cfm?fuseaction=vids.individual&videoid=6129 9226 Warner Music International, a leading company in national and international repertoire operates through numerous international affiliates and licensees in more than 50 countries. -
Nty and the People Who Shape It Platte
Community Platte County and the people who shape it Champions Keepsake Edition ow - 2019 is coming to an end. It didn’t feel that long ago like I was wit- nessing the start of spring, and then in what felt like a blink of an eye, we’re counting the days to Christmas and New Year’s Day. In early 2019, The Telegram started thinking about new and fun ways to Whighlight the people who make our community. Out of those conversations, “Commu- nity Champions” was born. Each week since April we have published in-depth human-interest profiles of people who live in Platte County and, are in a way, advocates for our community. We’ve come up with candidates ourselves, but have also gotten good ideas from readers. This special edition, the second “Community Champions” keepsake we’ve produced, recaps stories that published in The Telegram each week between early August and mid- October (profiles 15-28 in the series). Earlier this year, we published the first installment (profiles 1-14). Our plan is to publish these special editions every few months asthe 15) Jeff Gokie series continues. 25) Karina Perez Shout out to our advertisers for believing in community journalism and sponsoring good news. If you have ideas for profiles, send an email to [email protected]. Please include background and contact information. We’ll certainly consider them. Thanks to all our Champions who were willing to share their stories and put them- selves out there! Merry Christmas and Happy New Year! Matt Lindberg, Managing editor 16) Erin Nahorny Edition 2 26) Adam Roberts 17) Dillon Krueger 19) Nick Larson 21) Lynette Hogelin 23) Jessica Leffers 27) Megan Johnson 18) Kyle Jensen 20) Rob Gasper 22) Jenna Clark 24) Austin Olk 28) Charlie Bahr Sponsored by E2 | SATURDAY, DECEMBER 14, 2019 E2|Saturday, December 14, 2019 Community Champion Keepsake The Columbus Telegram Servant’s heart: Gokie takes pride in helping community MATT LINDBERG on weapons systems while at ideal spot to move the eatery to St.