Genetic Variation During Range Expansion: Effects of Habitat Novelty and Hybridization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biological Inventory and Evaluation of Conservation Strategies in Southwest Playa Wetlands
Biological Inventory and Evaluation of Conservation Strategies in Southwest Playa Wetlands Final Report to the Nebraska Game and Parks Commission and the Playa Lakes Joint Venture August 2007 Alison Banks Cariveau, Research Division Director Lacrecia Johnson, Playa Survey Project Leader Robert Sparks, Research Biologist Rocky Mountain Bird Observatory 14500 Lark Bunting Lane Brighton, CO 80603 303.659.4348 www.rmbo.org ROCKY MOUNTAIN BIRD OBSERVATORY The mission of the Rocky Mountain Bird Observatory (RMBO) is to conserve birds of the Rocky Mountains, Great Plains, and Intermountain West and the habitats on which they depend through research, monitoring, education, and outreach. RMBO practices a multi- faceted approach to bird conservation that integrates scientific research and monitoring studies with education and outreach programs to bring bird conservation issues to the public and other conservation partners. RMBO works closely with state and federal natural resource agencies, private landowners, schools, and other nonprofit organizations. RMBO accomplishes its mission by working in four areas: Research : RMBO studies avian responses to habitat conditions, ecological processes, and management actions to provide scientific information that guides bird conservation actions. Monitoring : RMBO monitors the distribution and abundance of birds through long-term, broad-scale monitoring programs that track population trends for birds of the region. Education : RMBO provides active, experiential, education programs for K-12 students in order to create an awareness and appreciation for birds, with the goal of understanding the need for bird conservation. Outreach : RMBO shares the latest information in land management and bird conservation practices with private landowners, land managers, and resource professionals at natural resource agencies. -

Watchable Wildlife Form
U.S. Fish & Wildlife Service Watchable Wildlife Bitter Lake National Wildlife Refuge Welcome Roundnose minnow Dionda episcopa Amphibians Bitter Lake National Wildlife Refuge is one Speckled chub Extrarius aestivalis Family Ambystomatidae – Mole Salamanders of New Mexico’s most important sanctuaries Plains minnow Hybognathus placitus Tiger salamander Ambystoma tigrinum Arkansas River shiner Notropis girardi and breeding grounds for migratory birds and Family Leptodactylidae – Tropical Frogs Rio Grande shiner Notropis jemezanus other wildlife. Established in 1937, the 24,500- Eastern barking frog Eleutherodactylus augusti Pecos bluntnose shiner Notropis simus pecosensis acre refuge is strategically located along the latrans Pecos River where the Chihuahuan Desert Fathead minnow Pimephales promelas Family Pelobatidae – Spadefoot Toads meets the Great Plains. The convergence of Family Catostomidae – Suckers Couch’s spadefoot toad Scaphiopus couchii these vastly different terrains has produced a River carpsucker Carpoides carpio diverse range of habitats, providing a home to New Mexico spadefoot toad Spea multiplicata a rich array of plant and animal life, including Family Ictaluridae – Catfishes Plains spadefoot toad Spea bombifrons Channel catfish Ictalurus punctatus a number of rare species. Family Bufonidae – Toads Bitter Lake NWR is best known for its Family Cyprinodontidae – Pupfish Woodhouse’s toad Bufo woodhousii spectacular variety of birds, particularly the Pecos pupfish Cyprinodon pecosensis Red-spotted toad Bufo punctatus Great Plains toad Bufo cognatus large migrations of ducks, geese, and cranes Family Fundulidae – Killifishes Texas toad Bufo speciosus during the fall and winter months. Many of Plains killifish Fundulus zebrinus Western green toad Bufo debilis insidior these creatures are drawn by the refuge’s Rainwater killifish Lucania parva wetlands, which offer an abundance of food as Family Hylidae – Treefrogs Family Poeciliidae – Livebearers well as ideal nesting habitat for some species. -

A Study of the Genus Scaphiopus: the Spade-Foot Toads
Great Basin Naturalist Volume 1 Number 1 Article 4 7-25-1939 A study of the genus Scaphiopus: the spade-foot toads Vasco M. Tanner Brigham Young University, Provo, UT Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Tanner, Vasco M. (1939) "A study of the genus Scaphiopus: the spade-foot toads," Great Basin Naturalist: Vol. 1 : No. 1 , Article 4. Available at: https://scholarsarchive.byu.edu/gbn/vol1/iss1/4 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. A STUI)^' ( )F THE CiI':NUS SCAIMIIOPUS^^) Thk Spade-foot Toads AWSCO M. TANNER Professor of Zoology and Entomolog\- Brigham Young University INTRODUCTION It is a little more than a Inindred years since Holbrook, 1836, erected the t;enus Scaplilopits descril)in,ij solifariiis, a species found along the Atlantic Coast, as the type of the genus. This species, how- ever, was described the year prevousl}-, 1835, by Harlan as Rana Jiolhrookii : thus holhrookii becomes the accepted name of the genotype. Many species and sub-species have been named since this time, the great majority of them, however, have been considered as synonyms. In this study I liave recognized the following species: holhrookii, hitrtcrii, couchii, homhifrons, haminondii, and interttiontamis. A vari- ety of holhrookii, described by Garman as alhiis, from Key West. Florida, may be a vaUd form ; but since I have had only a specimen or two for study. -

Investigating the Roles of Engineered Wetland
BUILDING BETTER WETLANDS FOR AMPHIBIANS: INVESTIGATING THE ROLES OF ENGINEERED WETLAND FEATURES AND MOSQUITOFISH (GAMBUSIA AFFINIS) ON AMPHIBIAN ABUNDANCE AND REPRODUCTIVE SUCCESS A Dissertation Presented to The Faculty of the Graduate School At the University of Missouri In Partial Fulfillment Of the Requirements for the Degree Doctor of Philosophy By CHRISTOPHER D. SHULSE Dr. Raymond D. Semlitsch, Dissertation Supervisor MAY 2011 The undersigned, appointed by the dean of the Graduate School, have examined the dissertation entitled BUILDING BETTER WETLANDS FOR AMPHIBIANS: INVESTIGATING THE ROLES OF ENGINEERED WETLAND FEATURES AND MOSQUITOFISH (GAMBUSIA AFFINIS) ON AMPHIBIAN ABUNDANCE AND REPRODUCTIVE SUCCESS Presented by Christopher D. Shulse A candidate for the degree of Doctor of Philosophy And hereby certify that, in their opinion, it is worthy of acceptance. Dr. Raymond D. Semlitsch Dr. James E. Carrel Dr. Lori S. Eggert Dr. Kathleen M. Trauth Dedicated to my wife, Tracy, and my children, Austin and Ashlee, whose love and support have sustained me through my endeavors. ACKNOWLEDGEMENTS I owe a great deal of gratitude to my graduate advisor, Dr. Ray Semlitsch, for his support, advice, and encouragement. I have learned much about amphibian conservation and scientific investigation from Ray; he is a skilled research scientist and devoted mentor to his students. The uplifting statement, “…I’d be lost but for the Grace of God,” has served as a subtle, but ever-present source of inspiration, within every e-mail correspondence from Ray. I thank the Semlitsch Lab, members both past and present, for their advice and encouragement during my infrequent visits to the lab. Although I have been very fortunate to complete my Ph.D. -

Species Lists
Appendix B: Sepcies Lists Appendix B: Species Lists In this appendix: Plants Mammals Birds Pollinators Fish and Mussels Reptiles and Amphibians Plants Scientific Name Common Name Abutilon theophrasti velvetleaf Acalypha ostryifolia pineland threeseed mercury Acalypha rhomboidea common threeseed mercury Acalypha virginica Virginia threeseed mercury Alliaria petiolata garlic mustard Amaranthus tamariscinus tall amaranth Ambrosia artemisifolia annual ragweed Ambrosia trifida great ragweed Ammannia coccinea valley redstem Amorpha brachycarpa leadplant Ampelopsis cordata heartleaf peppervine Amphicarpaea bracteata var. comosa American hogpeanut Amsonia illustris Ozark bluestar Anemone canadensis Canadian anemone Apocynum cannabinum Indian hemp Aristolochia tomentosa Woolly dutchman's pipe Artemisia annua sweet sagewort Asarum canadense Canadian wildginger Asclepias incarnata swamp milkweed Asclepias purpurascens purple milkweed Asclepias syriaca common milkweed Asclepias verticillata whorled milkweed Aster lateriflorus calico aster Aster pilosus hairy white oldfield aster Aster subulatus eastern annual saltmarsh aster Bergia texana Texas bergia Bidens cernua nodding beggerstick Bidens connata purplestem beggarticks Boehmeria cylindrica smallspike false nettle Callitriche terrestris terrestrial water-starwort Calystegia sepium hedge false bindweed Campsis radicans trumpet creeper Cardamine hirsuta hairy bittercress Carex crus-corvi ravenfoot sedge Carex hyalinolepis shoreline sedge, thinscale sedge Carex molesta troublesome sedge Cassia fasciculata -

Some Common Raptor Species in Montana
SOME COMMON RAPTOR SPECIES IN MONTANA Falconiformes Cathartidae Turkey Vulture (SR) Accipitridae Northern Harrier (Marsh Hawk) (SR) Northern Goshawk (PR) , Cooper's Hawk (Chicken Hawk) (PR) * Accipiter-like profile Sharp-shinned Hawk (PR) - and habits Golden Eagle (PR) Bald Eagle (PR) Osprey (SR) Red-tailed Hawk (SR) , Swainson's Hawk (SR) * Buteo-like profile Rough-legged Hawk (WR) * and habits Ferruginous Hawk (SR) - Falconidae Prairie Falcon (SR) , Peregrine Falcon (Duck Hawk) (SR) * Falco-like profile Merlin (Pigeon Hawk) (PR) * and habits American Kestrel (Sparrow Hawk) (SR) - Strigiformes Strigidae Great Gray Owl (PR) Great Horned Owl (PR) Long-eared Owl (PR) Short-eared Owl (PR) Western Screech-Owl (PR) Northern Saw-whet Owl (PR) Northern Pygmy-Owl (PR) Burrowing Owl (SR) __________________________________________ SR = summer resident PR = permanent resident WR = winter resident 49 Montana Raptors Raptors include birds with (1) strongly decurved bills for tearing flesh and (2) sharply decurved talons for grasping and killing prey. In Montana, the raptors include basically 2 orders: Falconiformes (Vultures and diurnal birds of prey) and Strigiformes (nocturnal birds of prey). With regard to their body and wing profile, most diurnal raptors can be categorized into cone of 3 basic groups, the name of which comes from the common genus in the group: (1) Accipiter-like birds have relatively short, rounded wings and long tails. This morphology is associated with a high degree of maneuverability; the birds commonly dart in and out among dense vegetation and take bird prey from the ground. (2) Falco-like birds have medium-sized wings that taper to a point, and fairly straight tails. -
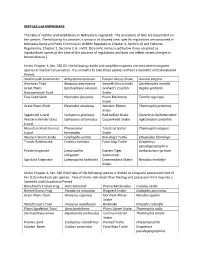
Nebraska Reptile and Amphibian Regulations
REPTILES and AMPHIBIANS The take of reptiles and amphibians in Nebraska is regulated. The provisions of take are dependent on the species. The following list provides a synopsis of allowed take; specific regulations are contained in Nebraska Game and Parks Commission Wildlife Regulations Chapter 4, Section 10 and Fisheries Regulations, Chapter 2, Sections 2, 6, and 9. (Scientific names used below those accepted as standardized names at the time of the issuance of regulations and does not reflect recent changes in Nomenclature.) Under Chapter 4, Sec. 010.02, the following reptile and amphibian species are considered nongame species in need of conservation. It is unlawful to take these species without a Scientific and Educational Permit. Smallmouth Salamander Ambystoma texanum Eastern Glossy Snake Arizona elegans American Toad Anaxyrus americanus Smooth Green Snake Liochlorophis vernalis Great Plains Gastrophryne olivacea Graham’s Crayfish Regina grahamii Narrowmouth Toad Snake Five-lined Skink Plestiodon fasciatus Plains Blackhead Tantilla nigriceps Snake Great Plains Skink Plestiodon obsoletus Western Ribbon Thamnophis proximus Snake Sagebrush Lizard Sceloporus graciosus Red-bellied Snake Storeria occipitomaculata Western Slender Glass Ophisaurus attenuatus Copperhead Snake Agkistrodon contortrix Lizard Mountain Short-horned Phrynosoma Terestrial Garter Thamnophis elegans Lizard hernandesi Snake Western Worm Snake Carphophis vermis Blanding’s Turtle Emydoidea blandingii Timber Rattlesnake Crotalus horridus False Map Turtle Graptemys pseudogeographica Prairie Kingsnake Lampropeltis Eastern Tiger Ambystoma trigrinum calligaster Salamander Speckled Kingsnake Lampropeltis holbrooki Diamondback Water Nerodia rhombifer Snake Under Chapter 4, Sec. 010.03A3 take of the following species is limited to a bag and possession limit of ten (10) individuals per species. Take of more individuals than the bag and possession limit requires a Scientific and Educational Permit. -

Researching Amphibian Numbers in Alberta (Rana): 2005 Provincial Summary
RESEARCHING AMPHIBIAN NUMBERS IN ALBERTA (RANA): 2005 PROVINCIAL SUMMARY Alberta Species at Risk Report No. 110 RESEARCHING AMPHIBIAN NUMBERS IN ALBERTA (RANA): 2005 PROVINCIAL SUMMARY Lisa Wilkinson and Gavin Berg Alberta Species at Risk Report No.110 Project Partners: ii Publication No. I/248 ISBN: 0-7785-4793-0 (Printed Edition) ISBN: 0-7785-4794-9 (Online Edition) ISSN: 1496-7219 (Printed Edition) ISSN: 1496-7146 (Online Edition) Illustration: Brian Huffman For copies of this report, contact: Information Centre – Publications Alberta Environment / Alberta Sustainable Resource Development Main Floor, Great West Life Building 9920 108 Street Edmonton, Alberta, Canada, T5K 2M4 Telephone (780) 422-2079 OR Visit our website at: http://www.srd.gov.ab.ca/fw/speciesatrisk/index.html This publication may be cited as: Wilkinson, L., and G. Berg. 2006. Researching Amphibian Numbers in Alberta (RANA): 2005 provincial summary. Alberta Species at Risk Report No.110. Alberta Sustainable Resource Development, Fish and Wildlife Division, Edmonton, AB. 29 pp. iii DISCLAIMER The views and opinions expressed are those of the authors and do not necessarily represent the policies of the Department or the Alberta Government. iv TABLE OF CONTENTS ACKNOWLEDGEMENTS ......................................................................................... VII EXECUTIVE SUMMARY .........................................................................................VIII 1.0 INTRODUCTION...................................................................................................... -

Bibliography of the Anurans of the United States and Canada. Version 2, Updated and Covering the Period 1709 – 2012
January 2018 Open Access Publishing Volume 13, Monograph 7 A female Western Toad (Anaxyrus boreas) from Garibaldi Provincial Park, British Columbia, Canada. This large bufonid occurs throughout much of Western North America. The IUCN lists it as Near Threatened because it is probably in significant decline (> 30% over 10 years) due to disease.(Photographed by C. Kenneth Dodd). Bibliography of the Anurans of the United States and Canada. Version 2, Updated and Covering the Period 1709 – 2012. Monograph 7. C. Kenneth Dodd, Jr. ISSN: 1931-7603 Indexed by: Zoological Record, Scopus, Current Contents / Agriculture, Biology & Environmental Sciences, Journal Citation Reports, Science Citation Index Extended, EMBiology, Biology Browser, Wildlife Review Abstracts, Google Scholar, and is in the Directory of Open Access Journals. BIBLIOGRAPHY OF THE ANURANS OF THE UNITED STATES AND CANADA. VERSION 2, UPDATED AND COVERING THE PERIOD 1709 – 2012. MONOGRAPH 7. C. KENNETH DODD, JR. Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida, USA 32611. Copyright © 2018. C. Kenneth Dodd, Jr. All Rights Reserved. Please cite this monograph as follows: Dodd, C. Kenneth, Jr. 2018. Bibliography of the anurans of the United States and Canada. Version 2, Updated and Covering the Period 1709 - 2012. Herpetological Conservation and Biology 13(Monograph 7):1-328. Table of Contents TABLE OF CONTENTS i PREFACE ii ABSTRACT 1 COMPOSITE BIBLIOGRAPHIC TRIVIA 1 LITERATURE CITED 2 BIBLIOGRAPHY 2 FOOTNOTES 325 IDENTICAL TEXTS 325 CATALOGUE OF NORTH AMERICAN AMPHIBIANS AND REPTILES 326 ADDITIONAL ANURAN-INCLUSIVE BIBLIOGRAPHIES 326 AUTHOR BIOGRAPHY 328 i Preface to Version 2: An Expanded and Detailed Resource. MALCOLM L. -

Herpetofaunal Inventory of the Missouri National Recreational River and the Niobrara National Scenic River
Herpetofaunal Inventory of the Missouri National Recreational River and the Niobrara National Scenic River Final Report By: Daniel D. Fogell and George R. Cunningham Woodhouse’s toad from James River Island, Missouri National Recreational River Submitted to: Northern Great Plains Inventory & Monitoring Coordinator National Park Service Mount Rushmore National Memorial Keystone, South Dakota 57751 December 1st, 2005 Contents Contents 1 Executive Summary 2 Introduction and Basic Design 4 Individual Park Results 7 Missouri National Recreational River 7 Abstract 7 Introduction and Methods 7 Results and Discussion 8 Recommendations for Management and Monitoring 11 Niobrara National Scenic River 13 Abstract 13 Introduction and Methods 13 Results and Discussion 14 Recommendations for Management and Monitoring 17 Conclusion 19 Recommendations for Monitoring 20 References Cited 22 Figures and Tables 23 Appendix 28 1 Executive Summary During 2003 and 2004 we inventoried the amphibian and reptile species of the Missouri National Recreational River (MNRR) in South Dakota and Nebraska and the Niobrara National Scenic River (NIOB) in Nebraska. Since prior surveys at NIOB had concentrated on the western portion of the park and surveys there were fairly adequate, this survey concentrated on the portion east of the Niobrara Valley Preserve, at the request of NPS. Also at NIOB, due to a complete absence of private landowner cooperation, surveys were limited to bridge crossings, adjacent roads, and one large property owned and managed by the Kansas Audubon Society. For MNRR, several large islands were included in the survey also at the request of NPS. Several inventory techniques we employed, including visual encounter surveys, frog and toad call surveys, turtle trapping, road surveys on roads that border the parks, seining for amphibian larvae, and the use of artificial cover objects as well as drift fences and pitfall traps. -

Mammals Reptiles Amphibians Fish Birds
Prairie Species List Key : Northern Grasshopper Mouse Walleye Perch (int) imperiled (i) Olive-backed Pocket Mouse Northern Pike rare (r) Prairie Vole Brook Stickleback endemic (e) Sagebrush Vole River Carpsucker Western Harvest Mouse White Sucker MAMMALS Western Jumping Mouse Bigmouth Buffalo Sucker White-footed Mouse Pumpkinseed Sunfish BATS SQUIRRELS *Fish listed above are likely to be Big Brown Bat Black-tailed Prairie Dog on the APR based on recent fish California Myotis Golden-mantled Ground Squirrel surveys directly on and near the Hoary Bat Least Chipmunk Reserve. Future surveys may Little Brown Myotis Richardson’s Ground Squirrel add or eliminate species from Long-eared Myotis Thirteen-lined Ground Squirrel this list. Long-legged Myotis Yellow-bellied Marmot Silver-haired Bat Townsend’s Big-eared Bat BIRDS UNGULATES Western Small-footed Myotis Big Horn Sheep CHICKADEES, CREEPERS Bison BEAVERS Black-capped Chickadee Elk Beaver Brown Creeper (r) Moose Mountain Chickadee Mule Deer CARNIVORES Red-breasted Nuthatch Pronghorn Badger White-tailed Deer Bobcat CUCKOOS Coyote Black-billed Cuckoo Least Weasel REPTILES Long-tailed Weasel DOVES Mink Bull Snake Mourning Dove Mountain Lion Common Sagebrush Lizard Rock Dove (r) Raccoon Eastern Racer Snake Red Fox Greater Short-horned Lizard FINCHES River Otter Painted Turtle American Goldfinch Short-tail Weasel Plains Gartersnake Cassin’s Finch Striped Skunk Prairie Rattlesnake Common Redpoll Snapping turtle Evening Grosbeak (r) GOPHERS Western Hog-nosed Snake Gray-crowned Rosy-finch Northern -

American Bison Status Survey and Conservation Guidelines 2010
American Bison Status Survey and Conservation Guidelines 2010 Edited by C. Cormack Gates, Curtis H. Freese, Peter J.P. Gogan, and Mandy Kotzman IUCN/SSC American Bison Specialist Group IUCN IUCN, International Union for Conservation of Nature, helps the world find pragmatic solutions to our most pressing environment and development challenges. IUCN works on biodiversity, climate change, energy, human livelihoods and greening the world economy by supporting scientific research, managing field projects all over the world, and bringing governments, NGOs, the UN and companies together to develop policy, laws and best practice. IUCN is the world’s oldest and largest global environmental organization, with more than 1,000 government and NGO members and almost 11,000 volunteer experts in some 160 countries. IUCN’s work is supported by over 1,000 staff in 60 offices and hundreds of partners in public, NGO and private sectors around the world. IUCN Species Programme The IUCN Species Programme supports the activities of the IUCN Species Survival Commission and individual Specialist Groups, as well as implementing global species conservation initiatives. It is an integral part of the IUCN Secretariat and is managed from IUCN’s international headquarters in Gland, Switzerland. The Species Programme includes a number of technical units covering Wildlife Trade, the Red List, Freshwater Biodiversity Assessments (all located in Cambridge, UK), and the Global Biodiversity Assessment Initiative (located in Washington DC, USA). IUCN Species Survival Commission The Species Survival Commission (SSC) is the largest of IUCN’s six volunteer commissions with a global membership of 8,000 experts. SSC advises IUCN and its members on the wide range of technical and scientific aspects of species conservation and is dedicated to securing a future for biodiversity.