Symptomatic Carriers of Hemophilia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Skin Injuries – Can We Determine Timing and Mechanism?
Skin injuries – can we determine timing and mechanism? Jo Tully VFPMS Seminar 2016 What skin injuries do we need to consider? • Bruising • Commonest accidental and inflicted skin injury • Basic principles that can be applied when formulating opinion • Abrasions • Lacerations }we need to be able to tell the difference • Incisions • Stabs/chops • Bite marks – animal v human / inflicted v ‘accidental’ v self-inflicted Our role…. We are often/usually/always asked…………….. • “What type of injury is it?” • “When did this injury occur?” • “How did this injury occur?” • “Was this injury inflicted or accidental?” • IS THIS CHILD ABUSE? • To be able to answer these questions (if we can) we need knowledge of • Anatomy/physiology/healing - injury interpretation • Forces • Mechanisms in relation to development, plausibility • Current evidence Bruising – can we really tell which bruises are caused by abuse? Definitions – bruising • BLUNT FORCE TRAUMA • Bruise =bleeding beneath intact skin due to BFT • Contusion = bruise in deeper tissues • Haematoma - extravasated blood filling a cavity (or potential space). Usually associated with swelling • Petechiae =Pinpoint sized (0.1-2mm) hemorrhages into the skin due to acute rise in venous pressure • medical causes • direct forces • indirect forces Medical Direct Indirect causes mechanical mechanical forces forces Factors affecting development and appearance of a bruise • Properties of impacting object or surface • Force of impact • Duration of impact • Site - properties of body region impacted (blood supply, -
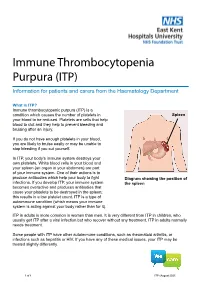
Immune Thrombocytopenia Purpura (ITP)
Immune Thrombocytopenia Purpura (ITP) Information for patients and carers from the Haematology Department What is ITP? Immune thrombocytopenic purpura (ITP) is a condition which causes the number of platelets in Spleen your blood to be reduced. Platelets are cells that help blood to clot and they help to prevent bleeding and bruising after an injury. If you do not have enough platelets in your blood, you are likely to bruise easily or may be unable to stop bleeding if you cut yourself. In ITP, your body’s immune system destroys your own platelets. White blood cells in your blood and your spleen (an organ in your abdomen) are part of your immune system. One of their actions is to produce antibodies which help your body to fight Diagram showing the position of infections. If you develop ITP, your immune system the spleen becomes overactive and produces antibodies that cause your platelets to be destroyed in the spleen; this results in a low platelet count. ITP is a type of autoimmune condition (which means your immune system is acting against your body rather than for it). ITP in adults is more common in women than men. It is very different from ITP in children, who usually get ITP after a viral infection but who recover without any treatment. ITP in adults normally needs treatment. Some people with ITP have other autoimmune conditions, such as rheumatoid arthritis, or infections such as hepatitis or HIV. If you have any of these medical issues, your ITP may be treated slightly differently. 1 of 8 ITP (August 2021) A normal platelet count is between 150 and 400 thousand million platelets per litre of blood. -

Duchenne Muscular Dystrophy in a Female Patient with a Karyotype of 46,X,I(X)(Q10)
Tohoku J. Exp. Med., 2010, 222, 149-153Karyotype Analysis of a Female Patient with DMD 149 Duchenne Muscular Dystrophy in a Female Patient with a Karyotype of 46,X,i(X)(q10) Zhanhui Ou,1 Shaoying Li,1 Qing Li,1 Xiaolin Chen,1 Weiqiang Liu1 and Xiaofang Sun1 1Institute of Gynecology and Obstetrics, The Third Affiliated Hospital of Guangzhou Medical College, Duobao Road, Guangzhou, China Duchenne muscular dystrophy (DMD) is a severe recessive X-linked form of muscular dystrophy caused by mutations in the dystrophin gene and it affects males predominantly. Here we report a 4-year-old girl with DMD from a healthy family, in which her parents and sister have no DMD genotype. A PCR-based method of multiple ligation-dependent probe amplification (MLPA) analysis showed the deletion of exons 46 and 47 in the dystrophin gene, which led to loss of dystrophin function. No obvious phenotype of Turner syndrome was observed in this patient and cytogenetic analysis revealed that her karyotype is 46,X,i(X)(q10). In conclusion, we describe the first female patient with DMD who carries a de novo mutation of the dystrophin gene in one chromosome and isochromosome Xq, i(Xq), in another chromosome. Keywords: Duchenne Muscular Dystrophy; de novo mutation; isochromosome Xq; karyotype; Turner syndrome Tohoku J. Exp. Med., 2010, 222 (2), 149-153. © 2010 Tohoku University Medical Press Duchenne muscular dystrophy (DMD) is a severe an uneventful pregnancy. At birth, her growth parameters recessive X-linked form of muscular dystrophy which is were normal. Her motor development was delayed: she characterized by rapid progression of muscle degeneration, could sit at 10 months and walk at 15 months, but fell down eventually leading to loss of ambulation and death. -

081999 Disseminated Intravascular Coagulation
The New England Journal of Medicine Current Concepts Systemic activation+ of coagulation DISSEMINATED INTRAVASCULAR COAGULATION Intravascular+ Depletion of platelets+ deposition of fibrin and coagulation factors MARCEL LEVI, M.D., AND HUGO TEN CATE, M.D. Thrombosis of small+ Bleeding and midsize vessels+ ISSEMINATED intravascular coagulation is and organ failure characterized by the widespread activation Dof coagulation, which results in the intravas- Figure 1. The Mechanism of Disseminated Intravascular Coag- cular formation of fibrin and ultimately thrombotic ulation. occlusion of small and midsize vessels.1-3 Intravascu- Systemic activation of coagulation leads to widespread intra- lar coagulation can also compromise the blood sup- vascular deposition of fibrin and depletion of platelets and co- agulation factors. As a result, thrombosis of small and midsize ply to organs and, in conjunction with hemodynam- vessels may occur, contributing to organ failure, and there may ic and metabolic derangements, may contribute to be severe bleeding. the failure of multiple organs. At the same time, the use and subsequent depletion of platelets and coag- ulation proteins resulting from the ongoing coagu- lation may induce severe bleeding (Fig. 1). Bleeding may be the presenting symptom in a patient with disseminated intravascular coagulation, a factor that can complicate decisions about treatment. TABLE 1. COMMON CLINICAL CONDITIONS ASSOCIATED WITH DISSEMINATED ASSOCIATED CLINICAL CONDITIONS INTRAVASCULAR COAGULATION. AND INCIDENCE Sepsis Infectious Disease Trauma Serious tissue injury Disseminated intravascular coagulation is an ac- Head injury Fat embolism quired disorder that occurs in a wide variety of clin- Cancer ical conditions, the most important of which are listed Myeloproliferative diseases in Table 1. -

Bleeds and Bruises in Children with Haemophilia
Bleeds and Bruises in CHildren WiTH HaeMOPHilia MusCle ANd/or JoiNt Bleeds Call the parent/guardian P.r.i.C.e. siGNs oF A serious HeAd Bleed P : Protection * Headache. Lower Limb: Take weight off the joint or muscle * drowsiness. Upper Limb: No carrying using affected arm * Nausea. r : rest * Vomiting. • Rest means rest! * unsteady Balance. • Try not to allow use of the joint or muscle where * irritability. possible. * Confusion. * seizures. i : ice * loss of consciousness. • Regular ice packs can help with pain & reduce swelling. • Put an ice pack over the affected area for 20 minutes. Repeat every two hours. DO NOT leave the ice pack on for more than 20 minutes siGNs oF A soFt tissue DO NOT place ice pack directly on skin (Use a tea Bleed towel/cold pack cover) * Bruising, discolouring of skin. C : Compression * Mild swelling. • Use an elasticated bandage to compress the affected area to reduce swelling. e : elevation • Elevate the affected limb to help reduce swelling. siGNs oF AN ABdoMiNAl • Keep the affected joint or muscle above the level of the Bleed heart. * Bloody, black or tar-like First Aid bowel motions. * red or brown urine. Mouth & Gum Bleeds * Pain. These can be hard to control because clots that form are * Vomiting of blood (blood washed away by saliva or knocked off by the tongue or food. Try giving the child an ice cube or ice pop to suck. may be red or black). These bleeds may need treatment by parents or the treatment centre. Nosebleeds siGNs oF BleediNG iNto tHe Tilt head forward and pinch the bridge of the nose below the bone for 10 - 20 minutes and / or put an ice-pack on JoiNts or MusCles the bridge of the nose for not more than 5 minutes. -

Isolated Plantar Vein Thrombosis Resembling a Corn with a Bruise
JE Hahm, et al pISSN 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 31, No. 1, 2019 https://doi.org/10.5021/ad.2019.31.1.66 CASE REPORT Isolated Plantar Vein Thrombosis Resembling a Corn with a Bruise Ji Eun Hahm, Kang Su Kim, Jae Won Ha, Chul Woo Kim, Sang Seok Kim Department of Dermatology, Kangdong Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Plantar vein thrombosis, rarely-reported disease, is usually or callus, plantar fibromatosis, or plantar verruca1. Among accompanied by pain and tenderness in the plantar region laborers, they may develop from excess pressure on the and should be differentiated from other dermatological con- bony prominences of the feet, repetitive uneven friction ditions causing plantar pain, such as hemorrhagic corn/cal- from footwear, or gait abnormalities. Plantar vein throm- lus, plantar epidermal cyst, verruca, or plantar fibromatosis. bosis is a rare condition causing plantar pain. The exact A 52-year-old man presented with a violaceous tender sub- cause of plantar vein thrombosis is yet unclear, but predis- cutaneous nodule overlying a hyperkeratotic plaque on his posing conditions, such as prior trauma, surgery, paraneo- sole. Initially, he thought it was a corn and applied keratolytic plastic syndromes, or coagulation disorders have been agents, which failed to work. Sonography revealed a well-de- described. To date, there is no established treatment ex- marcated mass with increased peripheral vascularity. His cept surgical excision, but reportedly, nonsteroidal anti-in- pain was relieved after a complete wide excision, which con- flammatory drug or heparin with elastic bandage is known firmed the mass to be plantar vein thrombosis after histo- to be effective for symptomatic control2-5. -

What Everyone Should Know to Stop Bleeding After an Injury
What Everyone Should Know to Stop Bleeding After an Injury THE HARTFORD CONSENSUS The Joint Committee to Increase Survival from Active Shooter and Intentional Mass Casualty Events was convened by the American College of Surgeons in response to the growing number and severity of these events. The committee met in Hartford Connecticut and has produced a number of documents with rec- ommendations. The documents represent the consensus opinion of a multi-dis- ciplinary committee involving medical groups, the military, the National Security Council, Homeland Security, the FBI, law enforcement, fire rescue, and EMS. These recommendations have become known as the Hartford Consensus. The overarching principle of the Hartford Consensus is that no one should die from uncontrolled bleeding. The Hartford Consensus recommends that all citizens learn to stop bleeding. Further information about the Hartford Consensus and bleeding control can be found on the website: Bleedingcontrol.org 2 SAVE A LIFE: What Everyone Should Know to Stop Bleeding After an Injury Authors: Peter T. Pons, MD, FACEP Lenworth Jacobs, MD, MPH, FACS Acknowledgements: The authors acknowledge the contributions of Michael Cohen and James “Brooks” Hart, CMI to the design of this manual. Some images adapted from Adam Wehrle, EMT-P and NAEMT. © 2017 American College of Surgeons CONTENTS SECTION 1 3 ■ Introduction ■ Primary Principles of Trauma Care Response ■ The ABCs of Bleeding SECTION 2 5 ■ Ensure Your Own Safety SECTION 3 6 ■ A – Alert – call 9-1-1 SECTION 4 7 ■ B – Bleeding – find the bleeding injury SECTION 5 9 ■ C – Compress – apply pressure to stop the bleeding by: ■ Covering the wound with a clean cloth and applying pressure by pushing directly on it with both hands, OR ■Using a tourniquet, OR ■ Packing (stuff) the wound with gauze or a clean cloth and then applying pressure with both hands SECTION 6 13 ■ Summary 2 SECTION 1: INTRODUCTION Welcome to the Stop the Bleed: Bleeding Control for the Injured information booklet. -

5.1.1 OCR Exambuilder
1. Thirty-three human blood group systems are known to exist. Two of these are the ABO blood group system and the Hh blood group system. Explain why a person whose blood group is AB expresses both A and B antigens on the surface of their red blood cells. [2] © OCR 2019. 1 of 42 PhysicsAndMathsTutor.com 2. Some varieties of maize plants have smooth kernels (seeds), whereas others have wrinkled kernels. This is a genetic trait. Varieties with smooth kernels are rich in starch and useful for making flour. A farmer has been given some smooth seeds all of the same unknown genotype. The farmer carries out a cross- breeding experiment using these seeds and some known to be heterozygous for this trait. The results are shown in Table 4.1. F1 phenotype Observed results Expected results Smooth 547 Wrinkled 185 Total 732 Table 4.1 The χ2 statistic is calculated in the following way: (i) Calculate the value of χ2 for the above data. Show your working. Answer _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ [2] (ii) Table 4.2 shows a critical values table. Degrees of freedom probability, p 0.90 0.50 0.10 0.05 1 0.016 0.455 2.71 3.84 2 0.211 1.386 4.61 5.99 3 0.584 2.366 6.25 7.81 4 1.064 3.357 7.78 9.49 Table 4.2 Using your calculated value of χ2 and Table 4.2 what conclusions should you make about the significance of © OCR 2019. -

Understanding Haemophilia
Understanding haemophilia Understanding haemophilia Contents Introduction 3 Haemophilia and your child 4 What is haemophilia? 5 What causes haemophilia? 5 Can females have haemophilia? 6 Carriers 8 Who is affected by haemophilia? 9 How severe is haemophilia? 9 Signs and symptoms of haemophilia 11 How is haemophilia diagnosed? 14 Diagnosis 14 Treatment 16 Port-a-cath 19 Managing joint bleeds with PRICE 19 Gene therapy 21 Possible complications of haemophilia 22 Inhibitors 22 Joint damage 22 Medical and dental treatment 23 Surgery Circumcision Dental care Medicines Vaccinations Bleeding disorder card Living with haemophilia 26 Sport and exercise 27 School, college and work 28 Travel 29 Pregnancy and haemophilia 30 Glossary of terms 32 About The Haemophilia Society 33 2 Understanding haemophilia Introduction This booklet is about haemophilia A and B. It gives a general overview of haemophilia and information on diagnosing, treating and living with the condition that we hope will answer your main questions. It has been written for people directly affected by haemophilia and for anyone interested in learning about haemophilia. If you are a parent and your child has recently been diagnosed with haemophilia you may be feeling quite overwhelmed. Remember, you’re not alone and many families are facing the same concerns and issues. Please do get in touch – we have lots of support and information available as well as services for parents and children. You can find out more via our website or Facebook pages, by emailing [email protected] or calling us on 020 7939 0780. The outlook is now the best it has ever been for people with haemophilia in the UK. -
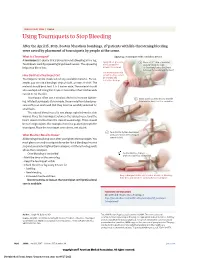
Using Tourniquets to Stop Bleeding
JAMA PATIENT PAGE | Trauma Using Tourniquets to Stop Bleeding After the April 15, 2013, Boston Marathon bombings, 27 patients with life-threatening bleeding were saved by placement of tourniquets by people at the scene. What Is a Tourniquet? Applying a tourniquet with a windlass device A tourniquet is a device that is placed around a bleeding arm or leg. Apply direct pressure 1 Place a 2-3” strip of material Tourniquets work by squeezing large blood vessels. The squeezing to the wound for about 2” from the edge helps stop blood loss. at least 15 minutes. of the wound over a long bone between the wound and the heart. Use a tourniquet only How Do I Put a Tourniquet On? when bleeding cannot be stopped and Tourniquets can be made out of any available material. For ex- is life threatening. ample, you can use a bandage, strip of cloth, or even a t-shirt. The material should be at least 2 to 3 inches wide. The material should also overlap itself. Using thin straps or material less than 2 inches wide can rip or cut the skin. Tourniquets often use a windlass device to increase tighten- 2 Insert a stick or other strong, straight ing. Inflated tourniquets (for example, those made from blood pres- item into the knot to act as a windlass. sure cuffs) can work well. But they must be carefully watched for small leaks. The injured blood vessel is not always right below the skin wound. Place the tourniquet between the injured vessel and the heart, about 2 inches from the closest wound edge. -

Haemophilia A
Haemophilia A Information for families Great Ormond Street Hospital for Children NHS Foundation Trust 2 Haemophilia A (also known as Classic Haemophilia or Factor VIII deficiency) is the most well-known type of clotting disorder. A specific protein is missing from the blood so that injured blood vessels cannot heal in the usual way. This information sheet from Great Ormond Street Hospital (GOSH) explains the causes, symptoms and treatment of Haemophilia A and where to get help. What is a clotting disorder? A clotting (or coagulation) disorder is a factor) turned on in order. When all of the medical condition where a specific protein factors are turned on, the blood forms a is missing from the blood. clot which stops the injury site bleeding Blood is made up of different types of any further. cells (red blood cells, white blood cells and There are a number of coagulation factors platelets) all suspended in a straw-coloured circulating in the blood, lying in wait to be liquid called plasma. Platelets are the cells turned on when an injury occurs. If any one responsible for making blood clot. When of the factors is missing from the body, the a blood vessel is injured, platelets clump complicated chemical reaction described together to block the injury site. They also above will not happen as it should. This can start off a complicated chemical reaction lead to blood loss, which can be severe and to form a mesh made of a substance called life-threatening. Each coagulation factor fibrin. This complicated chemical reaction is given a number from I to XIII – they are always follows a strict pattern – with each always written as Roman numerals – and clotting protein (known as a coagulation the effects of the missing factor will vary. -

Gene Therapy: the Promise of a Permanent Cure
INVITED COMMENTARY Gene Therapy: The Promise of a Permanent Cure Christopher D. Porada, Christopher Stem, Graça Almeida-Porada Gene therapy offers the possibility of a permanent cure for By providing a normal copy of the defective gene to the any of the more than 10,000 human diseases caused by a affected tissues, gene therapy would eliminate the problem defect in a single gene. Among these diseases, the hemo- of having to deliver the protein to the proper subcellular philias represent an ideal target, and studies in both animals locale, since the protein would be synthesized within the cell, and humans have provided evidence that a permanent cure utilizing the cell’s own translational and posttranslational for hemophilia is within reach. modification machinery. This would ensure that the protein arrives at the appropriate target site. In addition, although the gene defect is present within every cell of an affected ene therapy, which involves the transfer of a func- individual, in most cases transcription of a given gene and Gtional exogenous gene into the appropriate somatic synthesis of the resultant protein occurs in only selected cells of an organism, is a treatment that offers a precise cells within a limited number of organs. Therefore, only cells means of treating a number of inborn genetic diseases. that express the product of the gene in question would be Candidate diseases for treatment with gene therapy include affected by the genetic abnormality. This greatly simplifies the hemophilias; the hemoglobinopathies, such as sickle- the task of delivering the defective gene to the patient and cell disease and β-thalassemia; lysosomal storage diseases achieving therapeutic benefit, since the gene would only need and other diseases of metabolism, such as Gaucher disease, to be delivered to a limited number of sites within the body.