Telomere and Telomerase in Hematological Malignancies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduction of Human Telomerase Reverse Transcriptase to Normal Human Fibroblasts Enhances DNA Repair Capacity
Vol. 10, 2551–2560, April 1, 2004 Clinical Cancer Research 2551 Introduction of Human Telomerase Reverse Transcriptase to Normal Human Fibroblasts Enhances DNA Repair Capacity Ki-Hyuk Shin,1 Mo K. Kang,1 Erica Dicterow,1 INTRODUCTION Ayako Kameta,1 Marcel A. Baluda,1 and Telomerase, which consists of the catalytic protein subunit, No-Hee Park1,2 human telomerase reverse transcriptase (hTERT), the RNA component of telomerase (hTR), and several associated pro- 1School of Dentistry and 2Jonsson Comprehensive Cancer Center, University of California, Los Angeles, California teins, has been primarily associated with maintaining the integ- rity of cellular DNA telomeres in normal cells (1, 2). Telomer- ase activity is correlated with the expression of hTERT, but not ABSTRACT with that of hTR (3, 4). Purpose: From numerous reports on proteins involved The involvement of DNA repair proteins in telomere main- in DNA repair and telomere maintenance that physically tenance has been well documented (5–8). In eukaryotic cells, associate with human telomerase reverse transcriptase nonhomologous end-joining requires a DNA ligase and the (hTERT), we inferred that hTERT/telomerase might play a DNA-activated protein kinase, which is recruited to the DNA role in DNA repair. We investigated this possibility in nor- ends by the DNA-binding protein Ku. Ku binds to hTERT mal human oral fibroblasts (NHOF) with and without ec- without the need for telomeric DNA or hTR (9), binds the topic expression of hTERT/telomerase. telomere repeat-binding proteins TRF1 (10) and TRF2 (11), and Experimental Design: To study the effect of hTERT/ is thought to regulate the access of telomerase to telomere DNA telomerase on DNA repair, we examined the mutation fre- ends (12, 13). -

Genomic Correlates of Relationship QTL Involved in Fore- Versus Hind Limb Divergence in Mice
Loyola University Chicago Loyola eCommons Biology: Faculty Publications and Other Works Faculty Publications 2013 Genomic Correlates of Relationship QTL Involved in Fore- Versus Hind Limb Divergence in Mice Mihaela Palicev Gunter P. Wagner James P. Noonan Benedikt Hallgrimsson James M. Cheverud Loyola University Chicago, [email protected] Follow this and additional works at: https://ecommons.luc.edu/biology_facpubs Part of the Biology Commons Recommended Citation Palicev, M, GP Wagner, JP Noonan, B Hallgrimsson, and JM Cheverud. "Genomic Correlates of Relationship QTL Involved in Fore- Versus Hind Limb Divergence in Mice." Genome Biology and Evolution 5(10), 2013. This Article is brought to you for free and open access by the Faculty Publications at Loyola eCommons. It has been accepted for inclusion in Biology: Faculty Publications and Other Works by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. © Palicev et al., 2013. GBE Genomic Correlates of Relationship QTL Involved in Fore- versus Hind Limb Divergence in Mice Mihaela Pavlicev1,2,*, Gu¨ nter P. Wagner3, James P. Noonan4, Benedikt Hallgrı´msson5,and James M. Cheverud6 1Konrad Lorenz Institute for Evolution and Cognition Research, Altenberg, Austria 2Department of Pediatrics, Cincinnati Children‘s Hospital Medical Center, Cincinnati, Ohio 3Yale Systems Biology Institute and Department of Ecology and Evolutionary Biology, Yale University 4Department of Genetics, Yale University School of Medicine 5Department of Cell Biology and Anatomy, The McCaig Institute for Bone and Joint Health and the Alberta Children’s Hospital Research Institute for Child and Maternal Health, University of Calgary, Calgary, Canada 6Department of Anatomy and Neurobiology, Washington University *Corresponding author: E-mail: [email protected]. -

Telomeres.Pdf
Telomeres Secondary article Elizabeth H Blackburn, University of California, San Francisco, California, USA Article Contents . Introduction Telomeres are specialized DNA–protein structures that occur at the ends of eukaryotic . The Replication Paradox chromosomes. A special ribonucleoprotein enzyme called telomerase is required for the . Structure of Telomeres synthesis and maintenance of telomeric DNA. Synthesis of Telomeric DNA by Telomerase . Functions of Telomeres Introduction . Telomere Homeostasis . Alternatives to Telomerase-generated Telomeric DNA Telomeres are the specialized chromosomal DNA–protein . Evolution of Telomeres and Telomerase structures that comprise the terminal regions of eukaryotic chromosomes. As discovered through studies of maize and somes. One critical part of this protective function is to fruitfly chromosomes in the 1930s, they are required to provide a means by which the linear chromosomal DNA protect and stabilize the genetic material carried by can be replicated completely, without the loss of terminal eukaryotic chromosomes. Telomeres are dynamic struc- DNA nucleotides from the 5’ end of each strand of this tures, with their terminal DNA being constantly built up DNA. This is necessary to prevent progressive loss of and degraded as dividing cells replicate their chromo- terminal DNA sequences in successive cycles of chromo- somes. One strand of the telomeric DNA is synthesized by somal replication. a specialized ribonucleoprotein reverse transcriptase called telomerase. Telomerase is required for both -

Expression of Telomerase Activity, Human Telomerase RNA, and Telomerase Reverse Transcriptase in Gastric Adenocarcinomas Jinyoung Yoo, M.D., Ph.D., Sonya Y
Expression of Telomerase Activity, Human Telomerase RNA, and Telomerase Reverse Transcriptase in Gastric Adenocarcinomas Jinyoung Yoo, M.D., Ph.D., Sonya Y. Park, Seok Jin Kang, M.D., Ph.D., Byung Kee Kim, M.D., Ph.D., Sang In Shim, M.D., Ph.D., Chang Suk Kang, M.D., Ph.D. Department of Pathology, St. Vincent’s Hospital, Catholic University, Suwon, South Korea esis of gastric cancer and may reflect, along with Telomerase is an RNA-dependent DNA polymerase enhanced hTR, the malignant potential of the tu- that synthesizes TTAGGG telomeric DNA onto chro- mor. It is noteworthy that methacarn-fixed tissue mosome ends to compensate for sequence loss dur- cannot as yet substitute for the frozen section in the ing DNA replication. It has been detected in 85–90% TRAP assay. of all primary human cancers, implicating that the telomerase seems to be reactivated in tumors and KEY WORDS: hTR, Stomach cancer, Telomerase, that such activity may play a role in the tumorigenic TERT. process. The purpose of this study was to evaluate Mod Pathol 2003;16(7):700–707 telomerase activity, human telomerase RNA (hTR), and telomerase reverse transcriptase (TERT) in Recent studies of stomach cancer have been di- stomach cancer and to determine their potential rected toward gaining a better understanding of relationships to clinicopathologic parameters. Fro- tumor biology. Molecular analysis has suggested zen and corresponding methacarn-fixed paraffin- that alterations in the structures and functions of embedded tissue samples were obtained from 51 oncogenes and tumor suppressor genes, genetic patients with gastric adenocarcinoma and analyzed instability, as well as the acquisition of cell immor- for telomerase activity by using a TRAPeze ELISA tality may be of relevance in the pathogenesis of kit. -

Variation in the Anopheles Gambiae TEP1 Gene Shapes Local Population Structures of Malaria Mosquitoes
Variation in the Anopheles gambiae TEP1 Gene Shapes Local Population Structures of Malaria Mosquitoes D i s s e r t a t i o n Zur Erlangung des akademischen Grades D o c t o r r e r u m n a t u r a l i u m (Dr. rer. nat.) Im Fach Biologie eingereicht an der Lebenswissenschaftlichen Fakultät der Humboldt-Universität zu Berlin von BSc. (Biochemistry and Molecular Biology), MSc. (Biochemistry) Evans Kiplangat Rono Präsidentin der Humboldt-Universität zu Berlin: Prof. Dr.-Ιng. Dr. Sabine Kunst Dekan der Lebenswissenschaftlichen Fakultät: Prof. Dr. Bernhard Grimm Gutachter/innen: 1. Dr. Elena A. Levashina 2. Prof. Dr. Arturo Zychlinski 3. Prof. Dr. Susanne Hartmann Eingereicht am: Donnerstag, 04.05.2017 Tag der mündlichen Prüfung: Donnerstag, 29.06.2017 ii Zusammenfassung Abstract Zusammenfassung Rund eine halbe Million Menschen sterben jährlich im subsaharischen Afrika an Malaria Infektionen, die von der Anopheles gambiae Mücke übertragen werden. Die Allele (*R1, *R2, *S1 und *S2) des A. gambiae complement-like thioester-containing Protein 1 (TEP1) bestimmen die Fitness der Mücken, welches die männlichen Fertilität und den Resistenzgrad der Mücke gegen Pathogene wie Bakterien und Malaria- Parasiten. Dieser Kompromiss zwischen Reproduktion und Immunnität hat Auswirkungen auf die Größe der Mückenpopulationen und die Rate der Malariaübertragung, wodurch der TEP1 Lokus ein Primärziel für neue Malariakontrollstrategien darstellt. Wie die genetische Diversität von TEP1 die genetische Struktur natürlicher Vektorpopulationen beeinflusst, ist noch unklar. Die Zielsetzung dieser Doktorarbeit waren: i) die biogeographische Kartographierung der TEP1 Allele und Genotypen in lokalen Malariavektorpopulationen in Mali, Burkina Faso, Kamerun, und Kenia, und ii) die Bemessung des Einflusses von TEP1 Polymorphismen auf die Entwicklung humaner P. -

Fructose Causes Liver Damage, Polyploidy, and Dysplasia in the Setting of Short Telomeres and P53 Loss
H OH metabolites OH Article Fructose Causes Liver Damage, Polyploidy, and Dysplasia in the Setting of Short Telomeres and p53 Loss Christopher Chronowski 1, Viktor Akhanov 1, Doug Chan 2, Andre Catic 1,2 , Milton Finegold 3 and Ergün Sahin 1,4,* 1 Huffington Center on Aging, Baylor College of Medicine, Houston, TX 77030, USA; [email protected] (C.C.); [email protected] (V.A.); [email protected] (A.C.) 2 Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA; [email protected] 3 Department of Pathology, Baylor College of Medicine, Houston, TX 77030, USA; fi[email protected] 4 Department of Physiology and Biophysics, Baylor College of Medicine, Houston, TX 77030, USA * Correspondence: [email protected]; Tel.: +1-713-798-6685; Fax: +1-713-798-4146 Abstract: Studies in humans and model systems have established an important role of short telomeres in predisposing to liver fibrosis through pathways that are incompletely understood. Recent studies have shown that telomere dysfunction impairs cellular metabolism, but whether and how these metabolic alterations contribute to liver fibrosis is not well understood. Here, we investigated whether short telomeres change the hepatic response to metabolic stress induced by fructose, a sugar that is highly implicated in non-alcoholic fatty liver disease. We find that telomere shortening in telomerase knockout mice (TKO) imparts a pronounced susceptibility to fructose as reflected in the activation of p53, increased apoptosis, and senescence, despite lower hepatic fat accumulation in TKO mice compared to wild type mice with long telomeres. The decreased fat accumulation in TKO Citation: Chronowski, C.; Akhanov, is mediated by p53 and deletion of p53 normalizes hepatic fat content but also causes polyploidy, V.; Chan, D.; Catic, A.; Finegold, M.; Sahin, E. -
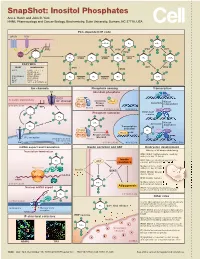
Snapshot: Inositol Phosphates Ace J
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

The Conserved Structure of Plant Telomerase RNA Provides the Missing Link for an Evolutionary Pathway from Ciliates to Humans
The conserved structure of plant telomerase RNA provides the missing link for an evolutionary pathway from ciliates to humans Jiarui Songa, Dhenugen Logeswaranb,1, Claudia Castillo-Gonzáleza,1, Yang Lib, Sreyashree Bosea, Behailu Birhanu Aklilua, Zeyang Mac,d, Alexander Polkhovskiya,e, Julian J.-L. Chenb,2, and Dorothy E. Shippena,2 aDepartment of Biochemistry and Biophysics, Texas A&M University, College Station, TX 77843; bSchool of Molecular Sciences, Arizona State University, Tempe, AZ 85287; cNational Maize Improvement Center of China, China Agricultural University, 100193 Beijing, China; dCollege of Agronomy and Biotechnology, China Agricultural University, 100193 Beijing, China; and eCenter of Life Sciences, Skolkovo Institute of Science and Technology, 121205 Moscow, Russian Federation Edited by Thomas R. Cech, University of Colorado Boulder, Boulder, CO, and approved October 24, 2019 (received for review September 4, 2019) Telomerase is essential for maintaining telomere integrity. Although transcribed by RNA polymerase III (Pol III) (6, 7). The La-related telomerase function is widely conserved, the integral telomerase protein P65 in Tetrahymena recognizes the 3′ poly-U tail of TR RNA (TR) that provides a template for telomeric DNA synthesis has and bends the RNA to facilitate telomerase RNP assembly (8, 9). diverged dramatically. Nevertheless, TR molecules retain 2 highly In contrast, fungi maintain much larger TR molecules (900 to conserved structural domains critical for catalysis: a template- 2,400 nt) that are transcribed by RNA polymerase II (Pol II) (3). proximal pseudoknot (PK) structure and a downstream stem-loop The 3′ end maturation of fungal TRs requires components of the structure. Here we introduce the authentic TR from the plant canonical snRNA biogenesis pathway and results in RNP assembly Arabidopsis thaliana, called AtTR, identified through next-generation sequencing of RNAs copurifying with Arabidopsis TERT. -

The Biochemical Activities of the Saccharomyces Cerevisiae Pif1 Helicase Are Regulated by Its N-Terminal Domain
G C A T T A C G G C A T genes Article The Biochemical Activities of the Saccharomyces cerevisiae Pif1 Helicase Are Regulated by Its N-Terminal Domain David G. Nickens y, Christopher W. Sausen y and Matthew L. Bochman * Molecular and Cellular Biochemistry Department, Indiana University, Bloomington, IN 47405, USA; [email protected] (D.G.N.); [email protected] (C.W.S.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 31 March 2019; Accepted: 20 May 2019; Published: 28 May 2019 Abstract: Pif1 family helicases represent a highly conserved class of enzymes involved in multiple aspects of genome maintenance. Many Pif1 helicases are multi-domain proteins, but the functions of their non-helicase domains are poorly understood. Here, we characterized how the N-terminal domain (NTD) of the Saccharomyces cerevisiae Pif1 helicase affects its functions both in vivo and in vitro. Removal of the Pif1 NTD alleviated the toxicity associated with Pif1 overexpression in yeast. Biochemically, the N-terminally truncated Pif1 (Pif1DN) retained in vitro DNA binding, DNA unwinding, and telomerase regulation activities, but these activities differed markedly from those displayed by full-length recombinant Pif1. However, Pif1DN was still able to synergize with the Hrq1 helicase to inhibit telomerase activity in vitro, similar to full-length Pif1. These data impact our understanding of Pif1 helicase evolution and the roles of these enzymes in the maintenance of genome integrity. Keywords: DNA helicase; Saccharomyces cerevisiae; Pif1; telomerase; telomere 1. Introduction DNA helicases are enzymes that couple DNA binding and ATP hydrolysis to unwind double-stranded DNA (dsDNA) into its component single strands [1]. -

DNA Bound by the Oxytricha Telomere Protein Is Accessible to Telomerase and Other DNA Polymerases DOROTHY E
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 405-409, January 1994 Biochemistry DNA bound by the Oxytricha telomere protein is accessible to telomerase and other DNA polymerases DOROTHY E. SHIPPEN*, ELIZABETH H. BLACKBURNt, AND CAROLYN M. PRICE0§ tDepartment of Microbiology and Immunology, University of California, San Francisco, CA 94143; and tDepartment of Chemistry, University of Nebraska, Lincoln, NB 68588 Contributed by Elizabeth H. Blackburn, August 25, 1993 ABSTRACT Macronuclear telomeres in Oxytricha exist as oftelomere protein in these two populations is not altered by DNA-protein complexes in which the termini of the G-rich additional nuclease treatment. strands are bound by a 97-kDa telomere protein. During The fragment of DNA bound by the majority of telomere telome'ic DNA replication, the replication machinery must protein molecules corresponds to the most terminal 13 or 14 have access to the G-rich strand. However, given the stability nucleotides of the T4G4T4G4 overhang (4). Dimethyl sulfate of telomere protein binding, it has been unclear how this is footprinting demonstrated that the complex formed between accomplished. In this study we investigated the ability of the telomere protein and the residual DNA fragment retains several different DNA polymerases to access telomeric DNA in the same DNA-protein contacts present at native telomeres Oxytricha telomere protein-DNA complexes. Although DNA (4). Thus, these telomeric DNA-protein complexes are useful bound by the telomere protein is not degraded by micrococcal substrates for in vitro investigations of telomere structure nuclease or labeled by terminal deoxynucleotidyltrnsferase, (10). In this study we have employed the DNA-protein this DNA serves as an efficient primer for the addition of complexes to analyze the interaction of protein-bound telo- telomeric repeats by telomerase, a specialized RNA-dependent meric DNA with components of the DNA replication ma- DNA polymerase (ribonucleoprotein reverse tanscriptase), chinery. -

Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt
International Journal of Molecular Sciences Article Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt 1,2, 1, 2 2 Stephanie Schwalm y, Martin Erhardt y, Isolde Römer , Josef Pfeilschifter , Uwe Zangemeister-Wittke 1,* and Andrea Huwiler 1,* 1 Institute of Pharmacology, University of Bern, Inselspital, INO-F, CH-3010 Bern, Switzerland; [email protected] (S.S.); [email protected] (M.E.) 2 Institute of General Pharmacology and Toxicology, University Hospital Frankfurt am Main, Goethe-University, Theodor-Stern Kai 7, D-60590 Frankfurt am Main, Germany; [email protected] (I.R.); [email protected] (J.P.) * Correspondence: [email protected] (U.Z.-W.); [email protected] (A.H.); Tel.: +41-31-6323214 (A.H.) These authors contributed equally to this work. y Received: 29 November 2019; Accepted: 12 February 2020; Published: 19 February 2020 Abstract: Ceramide kinase (CerK) is a lipid kinase that converts the proapoptotic ceramide to ceramide 1-phosphate, which has been proposed to have pro-malignant properties and regulate cell responses such as proliferation, migration, and inflammation. We used the parental human breast cancer cell line MDA-MB-231 and two single cell progenies derived from lung and bone metastasis upon injection of the parental cells into immuno-deficient mice. The lung and the bone metastatic cell lines showed a marked upregulation of CerK mRNA and activity when compared to the parental cell line. The metastatic cells also had increased migratory and invasive activity, which was dose-dependently reduced by the selective CerK inhibitor NVP-231. -

Mouse Tep1 Conditional Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Tep1 Conditional Knockout Project (CRISPR/Cas9) Objective: To create a Tep1 conditional knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Tep1 gene (NCBI Reference Sequence: NM_009351 ; Ensembl: ENSMUSG00000006281 ) is located on Mouse chromosome 14. 55 exons are identified, with the ATG start codon in exon 2 and the TGA stop codon in exon 55 (Transcript: ENSMUST00000006444). Exon 10 will be selected as conditional knockout region (cKO region). Deletion of this region should result in the loss of function of the Mouse Tep1 gene. To engineer the targeting vector, homologous arms and cKO region will be generated by PCR using BAC clone RP23-321A24 as template. Cas9, gRNA and targeting vector will be co-injected into fertilized eggs for cKO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Mice homozygous for a disruption in this gene show no obvious phenotype. No changes are seen in telomerase activity or telomere length. Exon 10 starts from about 19.88% of the coding region. The knockout of Exon 10 will result in frameshift of the gene. The size of intron 9 for 5'-loxP site insertion: 4878 bp, and the size of intron 10 for 3'-loxP site insertion: 990 bp. The size of effective cKO region: ~610 bp. The cKO region does not have any other known gene. Page 1 of 8 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele gRNA region 5' gRNA region 3' 1 10 11 12 13 55 Targeting vector Targeted allele Constitutive KO allele (After Cre recombination) Legends Exon of mouse Tep1 Homology arm cKO region loxP site Page 2 of 8 https://www.alphaknockout.com Overview of the Dot Plot Window size: 10 bp Forward Reverse Complement Sequence 12 Note: The sequence of homologous arms and cKO region is aligned with itself to determine if there are tandem repeats.