Of 27 PRODUCT MONOGRAPH Pr MAR-ACARBOSE Acarbose
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide As Batch Reaction
Pak. J. Chem. 5(3): 24-30, 2016 Full Paper ISSN (Print): 2220-2625 ISSN (Online): 2222-307X DOI: 10.15228/2016.v06.i01-2.p04 The Chemical Isomerization of Lactose to Lactulose by Using Sodium Hydroxide as Batch Reaction *Nahla T. K. Department of Food Sciences, College of Agriculture, University of Baghdad, Iraq E-mail: *[email protected] ABSTRACT Lactulose is a synthetic and non-absorbable disaccharide sugar containing glucose and fructose units. It is used for the treatment of constipation and hepatic encephalopathy. The purpose of this experiment was to synthesized lactulose in the presence of sodium hydroxide as a catalyst. The evaluation of lactulose synthesis was carried out at different concentration of sodium hydroxide (1, 2 and 3M) and the study was also conducted at different temperatures (30, 50, 70, and 90 °C). In addition, the kinetics of lactose isomerization with respect to the time also studied. Finally, it was concluded that the lactulose formation was determined by spectrophotometric method. The lactulose isomerization increased about more than 30.9% at 90 °C in the presence of 1M sodium hydroxide and 20 % lactose concentration. Keywords: Lactose, isomerization, lactulose, sodium hydroxide. 1. INTRODUCTION Lactulose is a synthetic sugar, which is not adsorbed by humans or rodents due to the lack of enzymes which catalyzes the disaccharide sugars. It can be generated by either alkaline isomerization of lactose via the Lobry de Bruyn - Alberda van Ekenstein rearrangement or by enzyme-catalyzed synthesis. Based on the first reaction, different process schemes for the preparation of lactulose have been developed. The enzymatic synthesis of lactulose can be carried out using different pathways with the transgalactosylation reaction being the most promising1. -

Concentration and Distribution of Sialic Acid in Human Milk and Infant Formulas1–3
Concentration and distribution of sialic acid in human milk and infant formulas1–3 Bing Wang, Janette Brand-Miller, Patricia McVeagh, and Peter Petocz Downloaded from https://academic.oup.com/ajcn/article/74/4/510/4737459 by guest on 23 September 2021 ABSTRACT 2- to 4-fold higher than those of other mammals, including chim- Background: In animal studies, sialic acid supplementation is panzees (4). Sialic acid is thought to play a role in the structural associated with increases of gangliosides in the brain and and functional establishment of synaptic pathways: >40% of improved learning ability. Only limited data are available on the sialic acid in the brain is found in the synaptosomal fraction and sialic acid content of human milk and infant formulas. contributes to the negative charge of the membrane (3). Because Objective: We compared the concentrations of oligosaccharide- most neurotransmitters are positively charged, sialic acid may bound, protein-bound, and free sialic acid in milk from mothers of assist neurotransmission by facilitating the binding of transmit- full-term and preterm infants and in a range of infant formulas. ter molecules to the synaptic membrane. Design: The milk from 20 and 14 mothers of full-term and Morgan and Winick (2) showed that exogenous sialic acid preterm infants (mean gestational age: 31 ± 3 wk), respectively, administered by intraperitoneal injection increased the produc- was collected at 4 stages of lactation (colostrum, transition, 1 mo, tion of ganglioside sialic acid in the brain and improved learning and 3 mo) and compared with 21 different infant formulas. ability in well-nourished and malnourished rat pups. -

Xorox Univerelty Microfilms
INFORMATION TO USERS This material was producad from a microfilm copy of the original document. While the moit advanced technological meant to photograph and reproduce thii document have been used, the quality it heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help ou understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to ob tain the mining page(s) or section, they are spliced into the film along w ith: adjacent pages, This may have necessitated cutting thru an image and dupli eating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image, fou will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning” the material. It is customary to begin photoi ig at the upper left hand comer of a large dieet and to continue photoi ig from left to right in equal sections with a small overlap. If necessary, sectioning is continued agein — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Lactulose Solution, Usp
LACTULOSE- lactulose solution VistaPharm, Inc ---------- LACTULOSE SOLUTION, USP Rx Only VP2051 04/10 DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g of other sugars). Also contains FD&C Yellow No. 6, purified water, USP and wild cherry flavoring. A minimal quantity of sodium hydroxide, NF is used to adjust pH when necessary. The pH range is 2.5 to 6.5. Lactulose is a colonic acidifier which promotes laxation.The chemical name for lactulose is 4-O-β-D- galactopyranosyl-D-fructofuranose. It has the following structural formula: C12H22O11 The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. -
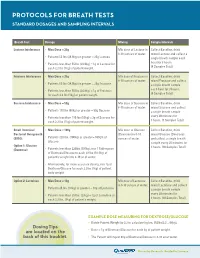
Protocols for Breath Tests Interpretation Help Standard Dosages and Sampling Intervals for Hydrogen/Methane Breath Tests
PROTOCOLS FOR BREATH TESTS INTERPRETATION HELP STANDARD DOSAGES AND SAMPLING INTERVALS FOR HYDROGEN/METHANE BREATH TESTS Breath Test Dosage Mixing Sample Intervals LACTOSE, FRUCTOSE OR SUCROSE INTOLERANCE: Lactose Intolerance • Max Dose = 25g Mix dose of Lactose in Collect Baseline, drink SUSPECTED POSITIVE: 8-10 ounces of water. mixed Lactose and collect a • Hydrogen (H ) Production Only: 20 ppm delta increase in Hydrogen from the lowest preceding value in the test. • Patients 55 lbs (24.9kg) or greater = 25g Lactose single breath sample each 2 hour for 3 hours. • Patients less than 55 lbs (24.9kg) = 1g of Lactose for • Methane (CH4) Production Only: 12 ppm delta increase in Methane from the lowest preceding value in the test. (4 Samples Total) each 2.2 lbs (1kg) of patient weight. • Hydrogen/Methane Both Produced: Add both H2 and CH4 values together in each sample. Review for a 15 ppm delta increase from the lowest preceding value. Fructose Intolerance • Max Dose = 25g Mix dose of Fructose in Collect Baseline, drink 8-10 ounces of water. mixed Fructose and collect • Patients 55 lbs (24.9kg) or greater = 25g Fructose a single breath sample each hour for 3 hours. SMALL INTESTINAL BACTERIAL OVERGROWTH (SIBO): • Patients less than 55 lbs (24.9kg) = 1g of Fructose (4 Samples Total) for each 2.2 lbs (1kg) of patient weight. SUSPECTED POSITIVE: Sucrose Intolerance • Max Dose = 50g Mix dose of Sucrose in Collect Baseline, drink OPTION 1: GLUCOSE (DEXTROSE) 8-10 ounces of water. mixed Sucrose and collect • Patients 110 lbs (50kg) or greater = 50g Sucrose • Hydrogen (H ) Production Only: 12 ppm delta increase in a single breath sample 2 every 30 minutes for Hydrogen from the lowest preceding value in the test. -

REVIEW the Role and Potential of Sialic Acid in Human Nutrition
European Journal of Clinical Nutrition (2003) 57, 1351–1369 & 2003 Nature Publishing Group All rights reserved 0954-3007/03 $25.00 www.nature.com/ejcn REVIEW The role and potential of sialic acid in human nutrition B Wang1* and J Brand-Miller1 1Human Nutrition Unit, School of Molecular and Microbial Biosciences, University of Sydney, NSW, Australia Sialic acids are a family of nine-carbon acidic monosaccharides that occur naturally at the end of sugar chains attached to the surfaces of cells and soluble proteins. In the human body, the highest concentration of sialic acid (as N-acetylneuraminic acid) occurs in the brain where it participates as an integral part of ganglioside structure in synaptogenesis and neural transmission. Human milk also contains a high concentration of sialic acid attached to the terminal end of free oligosaccharides, but its metabolic fate and biological role are currently unknown. An important question is whether the sialic acid in human milk is a conditional nutrient and confers developmental advantages on breast-fed infants compared to those fed infant formula. In this review, we critically discuss the current state of knowledge of the biology and role of sialic acid in human milk and nervous tissue, and the link between sialic acid, breastfeeding and learning behaviour. European Journal of Clinical Nutrition (2003) 57, 1351–1369. doi:10.1038/sj.ejcn.1601704 Keywords: sialic acid; ganglioside; sialyl-oligosaccharides; human milk; infant formula; breastfeeding Introduction promising new candidate is sialic acid (also known as The rapid growth and development of the newborn infant N-acetylneuraminic acid), a nine-carbon sugar that is a puts exceptional demands on the supply of nutrients. -

LACTULOSE SOLUTION USP - Lactulose Solution Hi-Tech Pharmacal Co., Inc
LACTULOSE SOLUTION USP - lactulose solution Hi-Tech Pharmacal Co., Inc. DESCRIPTION Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 1.2 g or less of other sugars). Lactulose solution contains potassium sorbate as an inactive ingredient. Lactulose is a colonic acidifier which promotes laxation. The chemical name for lactulose is 4-O-β-D-galactopyranosyl-D-fructofuranose. It has the following structural formula: The molecular weight is 342.30. It is freely soluble in water. CLINICAL PHARMACOLOGY Lactulose is poorly absorbed from the gastrointestinal tract and no enzyme capable of hydrolysis of this disaccharide is present in human gastrointestinal tissue. As a result, oral doses of lactulose reach the colon virtually unchanged. In the colon, lactulose is broken down primarily to lactic acid, and also to small amounts of formic and acetic acids, by the action of colonic bacteria, which results in an increase in osmotic pressure and slight acidification of the colonic contents. This in turn causes an increase in stool water content and softens the stool. Since lactulose does not exert its effect until it reaches the colon, and since transit time through the colon may be slow, 24 to 48 hours may be required to produce the desired bowel movement. Lactulose given orally to man and experimental animals resulted in only small amounts reaching the blood. Urinary excretion has been determined to be 3% or less and is essentially complete within 24 hours. -

SPC in English
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Lactulose-MIP 650 mg/ml oral solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of oral solution contains 650 mg of lactulose. For the full list of excipients, see section 6.1 3. PHARMACEUTICAL FORM Oral solution. Clear colourless or yellowish, viscous liquid. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Constipation, which cannot be influenced sufficiently by high-roughage diet or other general measures, as well as diseases requiring easy defecation. Prevention and treatment of portal systemic encephalopathy. 4.2 Posology and method of administration Posology Lactulose is taken orally. The dosage recommendations given below are meant as guidelines only, and hence are to be adapted to the requirements of the individual patients according to the severity and development of their condition. The quantities indicated can be measured with the accompanying measuring cup. Constipation: Adults and adolescents (14-17 years): The initial dose is 15-30 ml daily, taken either in a single dose (preferably in the morning) or in two divided doses. When the bowel movement begins, the dose should be reduced by half. If no response is seen in 3 days, the dosage may be raised to twice the initial dose. If still no response is observed a doctor should be consulted. If the patient has been treated with laxatives for a greater period of time, these should be discharged gradually. Children, daily dosage: Under 1 year: 5 ml 1-6 years: 10-15 ml 7-14 years 15 ml The dosage should be individually adjusted, producing bowel movements with soft stool. -

Probiotics and Prebiotics in the Elderly J M T Hamilton-Miller
447 Postgrad Med J: first published as 10.1136/pgmj.2003.015339 on 5 August 2004. Downloaded from REVIEW Probiotics and prebiotics in the elderly J M T Hamilton-Miller ............................................................................................................................... Postgrad Med J 2004;80:447–451. doi: 10.1136/pgmj.2003.015339 Probiotics (usually lactobacilli and bifidobacteria) and probiotics did not become popular until the public embraced the idea of functional foods prebiotics (non-digestible oligosaccharides) have been (‘‘foods that provide physiological benefits or shown to be useful in preventing certain disease conditions reduce the risk of chronic diseases, over and as well as possibly promoting specific aspects of health. In above their basic nutritional value’’) during the last decade of the 20th century. Metchnikoff’s the present review, the evidence from clinical trials for theory that gut flora contribute in some way to benefits from probiotics and prebiotics to elderly aging was supported in the 1950s, by the finding populations is presented and discussed, specifically in that germ-free animals live longer than their conventional counterparts.7 respect of three common conditions found in the elderly. Today, probiotics are familiar to the public as Both probiotics and prebiotics may be helpful in the components of bioyoghurts and dietary malnutrition, particularly in lactose intolerance and calcium supplements, are widely available, and exten- sively purchased. In 1997, Europeans spent the absorption, and in constipation. Probiotics have been equivalent of almost $900 million on probiotic shown clearly to boost immunity in the elderly, but the yoghurts and milks, and this market is growing clinical significance of this remains to be clarified. -

Lactulose, Disaccharides and Colonic Flora Clinical Consequences
Drugs 1997 Ju n: 53 (6): 930-942 REVIEW ARTICLE 00 12-6667/97/rJ:XJO-O<I3O/S13.00/0 © Ad isInterna tional lim ited . AUrights reserved . Lactulose, Disaccharides and Colonic Flora Clinical Consequences Mette Rye Clausen and PerBrebech Mortensen Department of Medicine CA, Division of Gastroenterology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark Contents Summary . 930 1. Dietary Carbohydrate . 931 1.1 Lactulose . 931 2. Colonic Fermentation and Producti on of Short-Chain Fatty Acids (SCFA) 931 2.1 Fermentation of Lactulose . 932 3. Lactulose in the Management of Constipation ........ 933 3.1 Colonic Compensation in Car bohydrate Malabsorption 933 3.2 Fermentation Capac ity Threshold: Individual Variability . 934 3.3 Does Lactulose Exert Its Laxative Effect Through the Osmotic Activity of Unferme nted Carbohydrate? . 935 3.4 Capac ity of the Colon to Absorb Fluid . 936 3.5 Possible Me chanisms of Action of Lactulose in the Treatment of Constipation. 936 4. Lac tulose in the Management of Hepatic Encephalopathy . 937 4.1 Effects of Lac tulose on Ammonia and Nitrogen Metabolism . 937 4.2 Role of Short- and Medium-Chain Fatty Acids in Hepatic Encephalopathy . 938 4.3 Effect of Lactulose on Colon ic SCFA Production in Hepatic Encephalopathy 939 4.4 Possible Mechanisms of Action of Lactulose in the Treatment of Hepatic Encephalopathy 940 5. Conclusions.................................. 940 Summary Lactulose is one of the mos t freq uently utilised age nts in the treatment of constipation and hepatic ence pha lopathy because of its efficacy and good safe ty profile. The key to understanding the possible modes ofaction by which lactulose ac hieves its therapeutic effects in these diso rders lies in certain pharmacological phe nomena: (a) lactulose is a sy nthetic disaccharide that does not occur naturally; (b) there is no disaccharidase on the microvillus membrane of enterocytes in the human small intesti ne that hydrolyses lactulose; and (c) lactulose is not absorbed from the small intestine. -

Hydrogen/Methane Breath Tests
Green Spring Station Out-Patient Center 2360 W. Joppa Rd. Suite 206, Lutherville, MD 21093 Kerrie Hendren, RN BSN 410-583-2614 HYDROGEN/METHANE BREATH TESTS What is a BREATH TEST? Information for physicians and patients. (H2. Hydrogen and CH4. Methane) Hydrogen and/or Methane gas in the body are produced from intestinal bacteria. Bacteria, normally in the large intestine, produce hydrogen or methane through fermentation of carbohydrates. Substrates containing these carbohydrates, like Lactulose, is given orally to test for small intestinal bacterial presence. Some of the hydrogen or methane produced from the bacterial fermentation causes bloating, abdominal discomfort or diarrhea. The gases are absorbed by the intestinal mucosa and enter the vasculature and transported to the lungs. The gases are then exhaled through normal breathing. These are collected in a bag for immediate analysis. In Small Intestinal Bacterial Overgrowth (SIBO), bacteria exist in the small intestine. Lactulose, when used as the challenge dose, is poorly absorbed in the gastrointestinal tract making it the perfect substrate to test for bacterial overgrowth throughout the length of the small bowel (21 feet). NOTE: If lactose or fructose is given as substrate, bacteria compete with the natural digestive process and metabolize the sugar before it is absorbed and may produce an early rise in breath hydrogen. Dedicated intolerance testing for these sugars are performed separately. In Lactose and Fructose intolerance, the individual has a deficiency in the enzymes needed for its absorption. Normally, it is broken down in the small intestine, absorbed, and very little lactose or fructose reach the large intestine. If it reaches the colon in its raw form, it is metabolized by colonic bacteria producing a large amount of gas which can be measured distally in the breath sample. -

Diet for Those with Symptomatic Small Bowel Bacterial Overgrowth
Diet for Those with Symptomatic Small Bowel Bacterial Overgrowth What is small bowel bacterial overgrowth (SBBO or SIBO for short)? We all have bacteria in our intestines, but some people have too much. These extra bacteria can cause problems. Good movement of food through the small bowel helps avoid this problem, and so does having normal amounts of stomach acid. So, people with slow bowel motility or who make too little gastric acid are at risk for bacterial overgrowth. What are the symptoms? Symptoms include gas, abdominal pain, distention, bloating, fullness, diarrhea, nausea, and/or pain after eating foods that are high in sugars, like sodas, sweets and desserts, or high in fiber, such as pinto beans, kidney beans, bran cereals, etc. How does what I eat make my symptoms worse? When there are too many bacteria high up in your intestine near your stomach, they get “first dibs” on the food that you eat – instead of you! The bacteria use your food for fuel, and they make gas in the process. This is what makes you uncomfortable. Foods that do not contain carbohydrates or fiber do not usually cause problems. This includes meats (beef, pork, lamb, venison); poultry (chicken, turkey, duck); fish and shellfish; eggs; and butter, oils, and hard cheeses. How to best use this diet: Look over the list of foods below and cut out the foods and drinks you eat a lot of. Start by at least cutting out concentrated sugars and sweets. If you feel a lot better, that may be all you need to do.