Inflammatory and Antioxidant Activities of Scyphocephalium Ochocoa Warb
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Item Box Subject Author Title Exps Pages Size Inches Pub. Date Grand
Item Box Subject Author Title Exps Pages Size Inches Pub. Date Grand Total: 3, 139, 369, 104, 343, 159, [and the 210 Namibian 51, 612, 191, 21, 44, 1, 39, 95, 428, docs so far is 2809] (2599) Central Africa:3 1 Central Africa—General Economics UNECA Subregional Strategies 19 32 8x11.5 Hints to Businessmen Visiting The London Board of 2 Central Africa—General Economics Congo (Brazzaville), Chad, Gabon 19 32 4.75x7.125 Trade and Central African Republic Purpose and Perfection Pottery as 3 Central Africa—General Art The Smithsonian Institution 3 4 8x9.25 a Woman's Art in Central Africa Botswana:139 National Institute of Access to Manual Skills Training in 1 Botswana—Bibliographies Bibliography Development and Cultural Botswana: An Annotated 9 13 8x11.5 Research Bibliography Social Thandiwe Kgosidintsi and 2 Botswana—Bibliographies Sciences—Information Publishing in Botswana 2 2 8.5x11 Neil Parsons Science National Institute of 3 Botswana—Bibliographies Bibliography Development Rearch and Working Papers 5 8 5.75x8.25 Documentation University of Botswana and Department of Library Studies 1 Botswana—Social Sciences Social Sciences 28 25 8.25x11.75 Swaziland Prospectus Social Refugees In Botswana: a Policy of 2 Botswana—Social Sciences United Nations 3 7 4.125x10.5 Sciences—Refugees Resettlement Projet De College Exterieur Du 3 Botswana—Social Sciences Social Sciences unknown 3 3 8.25x11.75 Botswana Community Relations in Botswana, with special reference to Francistown. Statement 4 Botswana—Social Sciences Social Sciences Republic of Botswana Delivered to the National Assembly 4 5 5.5x8 1971 by His Honor the Vice President Dt. -

Report on the April, 1995 Cites Implementation Workshop in Bangladesh
REPORT ON THE APRIL, 1995 CITES IMPLEMENTATION WORKSHOP IN BANGLADESH Prepared By John Brooks, Special Agent-Law Enforcement Sheila Einsweiler, Senior Wildlife Inspector-Law Enforcement Mark Phillips, Biologist-Office of Management Authority Supported By The Bangladesh Office of the Chief Conservator of Forest USAID's United States-Asia Environmental Partnership U.S. Fish & Wildlife Service August 9, 1995 A PROGRAM OF THE OFFICE OF INTERNATIONAL AFFAIRS U.S. FISH AND WILDLIFE SERVICE EXECUTIVE SUMMARY The United States-Asia Environmentai Partnership (USAEP) of the U.S. Agency for International Development and the Office of International Affairs of the U.S. Fish and Wildlife Service have joined in partnerships with the wildlife management agencies of Bangladesh, India, Nepal, indonesia and The Philippines to present workshops on the imple entation of the Convention on International Trade in Endangered Species of Flora and Fauna (CITES). The purpose of this program is to provide basic, practical training in the provisions of CITES, the reasons why they were developed, and their irmplementation. ATn emphasis is placed on CITES administration, w-idlife inspection, law enforcement investigation technicues and species identification. The Bangladesh workshop was the second to be presented in the series of five. In Bangladesh the partnership was formed with the Ministry of Environment and Forest. The workshop was held at the Bana Bhaban, Mohakhali, Dhaka from April 22 to 26, 1995. The Fish Wildlife and Service instructor team was in Bangladesh from April 17 to 29. The workshop was presented to twenty-four participants drawn from the Office of the Chief Conservator of Forest, Police, Customs, and one member of a non-governmental organization. -

Marcus Toothpaste
1 : l^f^^' ' A " ' ' 7 'THE -CRANFOBD' * >' . meeting at the home of Brendji Rothbard has been nominate far Conley, 14 Woods B«le road, some president; Mr* Marvin Kat*v Mrs. ians of the girls waxed' decorated eggs Harold Robinson and Mrs. Robert club, C. a MacFarland, Charles and prepared the basket, with the Grimshaw, vice-presidents; Mrs. Plans for- the establishment of idp of Mrs. Joseph Potts and Mrs a new ail-induslve fund-raising Bailey, Charles Wister, and C. B.pier, Steven Ulichny, Craig Sny- WUI|am Yuin. recording secretary; Smith, *- of Westfldd, and Winder, Richard Jackson, and Dan Albert MandeL, Meanwhile, the Mrs, Julius CtrMljC^trMlj correspoudcorrspoud- lrgahuatlon- for Cranford; weri KENILWORTH jther girls of the troop did'the M lli Sith outlined tar the Hotary, Club at Jones of the Dunellen dub. Otto Knauer. - ..••:. .•^.-.,T. " :. ._•:»'•_• GARWOOD ing secretary; Mrs. William Smith, remote, organizer of the Union Awards were, presented to the CRANFOR] At Luncheon marketing with the assistance of its weekly luncheon nw<tlng at I : The Cranford Newcomers Club Mrs. S. Gmelin and Mrs. Meredith treasurer. ;'••'•'•"•: - ""'• Junior .College silver anniversary following:.' • ' ' > <"'•' SBImd «a-anoo« dMa BMO matter at 3 Sections, 24 „___ _ * at itsConley, using funds earned by the Assisting Mrs. Pollack on theCranford Methodist Church last development ' campaignpg, , was a Allen Steinberg, gold arrow on CRAN^RD, NEW JERSEY, THURSDAY, i^RIL 24, 1958 , Tte Bolt OfOea at Cnalord, M. J. monthly luncheon meeting last nominating committee were, Mrs; Thursday by Joseph Kohn, chair- p Dr. Kenneth MacKay. Brownies.'' •" • .,,..'.'. ',.•'. '•'•:.' •..• ; • Kenneth Morris, Mrs; Joseph AU- guest of. -

Music Supervisor, Was
^^^^^^^^^^^^^^^^^^^^jgg Homes Sold Here ..- • '• " • •'• i •;:.-:.'>-fe;i> pi ^^^ w^&^§^f ;.•• Aa an example offon»ei»ftt* Holyt C^ininuQdoTi vdll be Cele- lowing fcomes In Crahford: A Boy Scout troops of the;.Cran^ :neefaltud'.plaoenwnt being done brated, with;' the (Rev. WiHiim H. one-family at 701 Spri«field ave- , avenue, was awar Contribute 'Nttt<w«ek'wUlbe *T&w«r tfottfSau*r;. Miss Elizabeth L. Durrell, the fo^wing'day confirmation of torji.andKenilworth districts will 1 nue to Geza Demeter for Charles entertain their- parents and; fellow Junior .genetal athievemej Contribute p&jfcHc Health Nurse W«*k," epos* school mu^; J^J^**. KtKatbryib iJ Vpp, fafa placement ^/r«peivjfedL'.:'-r -' -/Niefeanck,.pastc"r, officiating^ at the at &e graduation United State* EmjuoywientBerviotjyweB , StoAebridge; a one-family ai 103scouts with a play depicting a to thej iibib the NaHonal and' Stale school and attendance mine; Hti • BJr.Friemanako cite* the place- 11 a. m. service at Calvary Lutti- to the Hdfeert T, FrienanFri , LLabob rXbSfr Preston avenue to Phlbp Green- at the Boy Scout Camp, to be fltt f Ihbll'H^th Alice" I. Doran. aodal faysiene ment of a plant manager In the era! tiosplial BephMentatlve of tbe local eran Churdfi this'filunday. Sun- span for; Sidney Nunn J a ofre-fam- sentedatSt Michael's School ^ Cancer in cooperation with the nurse ' and lecturer <rf.4lie State New Srunswicfc.Biwa by tile local lt kT' Cancer Fund ,dte»lthe placing <rf«Hirt of offleeaiaa. day SAwl classes Will be held et lly at 6I« Orange avenue to George on TWday,- April 12. -

Atoll Research Bulletin No. 392 the Flora of Nauru Rr
ATOLL RESEARCH BULLETIN NO. 392 THE FLORA OF NAURU RR THAMAN, F.R FOSBERG, EL MANNER AND D.C. HASSALL ISSUED BY NATIONAL MUSEUM OF NATURAL J!WTORY SMllTJ!WNIAN INSTlTUTION WASHINGTON, D.C, USA FEBRUARY 1994 DEDICATION We dedicate this Flora of Nauru to Joseph Detsimea Audoa, his family and the people of the Republic of Nauru who have had their precious island and its flora destroyed and degraded as a result of wars and exploitation beyond their control. ACKNOWLEDGEMENTS The authors would like to acknowledge, in particular, the late Honorable Joseph Detsimea Audoa, the Minister of Health and Education at the time of the commencement of the study and later Minister of Justice in the Government of Nauru, who, because of his vision and commitment to the culture and environment of Nauru, initiated and provided the financial support for the study of the flora of Nauru. He was particularly concerned that the plants of Nauru and their cultural uses be recorded before such knowledge was lost. We also acknowledge Mr. Lisle Newby, the then Director of Education, who, along with Joe Audoa, were the main supporters of the project, and who provided valuable logistical support throughout. Special thanks are also given to our main local informants and assistants, the Reverend James Aingimea and the late Henry Michael Heine; and to Daphne Fotu, Jacob Gabwinare, Katarina Satto, Kenia Raidinen, Reynold Capelle, Eda Adam and Montiba Star, our main informants in relation to the cultural uses and Nauruan names of plants. Our thanks also go to the Honorable Lawrence Stephen, Minister of Education during part of the project; Obera Menke, Robert Kaierua, Leo Keke, Delilah Capelle, Eddie Borak, John Healy, Gary Bailey, Dennis and Ria Berdinner, Julie Olsson, Dennis Ketner, Sio Fotu, Pine Harrison, John Brechtefeld, Rene Harris, Porthos Bop, Jacob Aroi, Leon Thompson, Benjamin Morgan, Iosefa Elisala and Teaora Tabanou, all of whom contributed in some way to the success of the study. -

530 CIAO BRAMPTON on ETHNIC AM 530 N43 35 20 W079 52 54 09-Feb
frequency callsign city format identification slogan latitude longitude last change in listing kHz d m s d m s (yy-mmm) 530 CIAO BRAMPTON ON ETHNIC AM 530 N43 35 20 W079 52 54 09-Feb 540 CBKO COAL HARBOUR BC VARIETY CBC RADIO ONE N50 36 4 W127 34 23 09-May 540 CBXQ # UCLUELET BC VARIETY CBC RADIO ONE N48 56 44 W125 33 7 16-Oct 540 CBYW WELLS BC VARIETY CBC RADIO ONE N53 6 25 W121 32 46 09-May 540 CBT GRAND FALLS NL VARIETY CBC RADIO ONE N48 57 3 W055 37 34 00-Jul 540 CBMM # SENNETERRE QC VARIETY CBC RADIO ONE N48 22 42 W077 13 28 18-Feb 540 CBK REGINA SK VARIETY CBC RADIO ONE N51 40 48 W105 26 49 00-Jul 540 WASG DAPHNE AL BLK GSPL/RELIGION N30 44 44 W088 5 40 17-Sep 540 KRXA CARMEL VALLEY CA SPANISH RELIGION EL SEMBRADOR RADIO N36 39 36 W121 32 29 14-Aug 540 KVIP REDDING CA RELIGION SRN VERY INSPIRING N40 37 25 W122 16 49 09-Dec 540 WFLF PINE HILLS FL TALK FOX NEWSRADIO 93.1 N28 22 52 W081 47 31 18-Oct 540 WDAK COLUMBUS GA NEWS/TALK FOX NEWSRADIO 540 N32 25 58 W084 57 2 13-Dec 540 KWMT FORT DODGE IA C&W FOX TRUE COUNTRY N42 29 45 W094 12 27 13-Dec 540 KMLB MONROE LA NEWS/TALK/SPORTS ABC NEWSTALK 105.7&540 N32 32 36 W092 10 45 19-Jan 540 WGOP POCOMOKE CITY MD EZL/OLDIES N38 3 11 W075 34 11 18-Oct 540 WXYG SAUK RAPIDS MN CLASSIC ROCK THE GOAT N45 36 18 W094 8 21 17-May 540 KNMX LAS VEGAS NM SPANISH VARIETY NBC K NEW MEXICO N35 34 25 W105 10 17 13-Nov 540 WBWD ISLIP NY SOUTH ASIAN BOLLY 540 N40 45 4 W073 12 52 18-Dec 540 WRGC SYLVA NC VARIETY NBC THE RIVER N35 23 35 W083 11 38 18-Jun 540 WETC # WENDELL-ZEBULON NC RELIGION EWTN DEVINE MERCY R. -
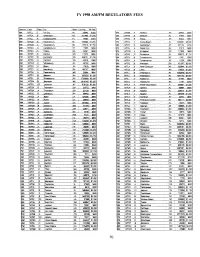
FY 1998 Amlfm REGULATORY FEES
FY 1998 AMlFM REGULATORY FEES Service ICalls IClass: City I State ! C,tv Poo ! 98 Fee AM IWTCJ IC Tell C,lv IN 25896 $300 FM WTKM ,A Harttord WI 34450 $600 AM IWTCK IB Greensboro INC 261488 $1.250 AM IWTKN :B !Oaleville iAl 47945 $600 AM WTCl 'B ,Chattahoochee ' FL 16980 $300 AM !WTKO IB nhaca 'NY 83442 $800 AM IWTCM B i Traverse City 'MI 183936 $1.250 FM IWTKS C Cocoa BeaCh Fl 1495391 S400C FM IWTCM C '. Traverse City 'MI 137013 1 $1.750 AM IWTKT 10 GeorQetown ,KY 267132 $750 AM IWTCO ,C ' Campbellsville KY 17855' $200 FM IWTKU A ,Ocean City !NJ 37210 $600 f!!L. IWTCQ iA IVidalia IGA 23391 " $600 FM IWTttCN 'A Bridgeport INY 117856'_~ AM 'WTCR IB IKenova ',WV 117372 $800 FIJI 'WTKX ICI 1Pensacola 1Fl 328372 $1750 FM IWTCR B 1Huntlhoton IWV 269721 $1750 AM ,WTKY IB 'TomOklnsville IKY 11301 $300 I AM ,WTCS !C Fairmont WV 43018, $300 FIJI IWTKY ,A I Tompkmsville KY 11226' $300 I AM !WTCW 0 ' Whitesbur<> 'KY 357021 $400 AM WTKZ IB 'Allentown IPA i 421397: $2.000 FM WTCX IA Rllxm WI 178191 $300 AM IWTLA IB :North Syracuse INY 335849 $1,250 1 AM ,WTCY CIHamsburo IPA 183737 $600 AM 'WTLB 18 Utica NY 178233 $1,250 FM WTOK A IFederalSDuro IMO 25664 $600 AM WTLC IB 'j Indtanacoils liN 10829581 $3,250 AM IWTOY B Mad,son IWI I 333223 I $1,250 FM WTLC 'B I,ndlanapells liN 10641031 $4,000 AM IWTEL B Phlladeloh,a IPA I 3700908! $3,250 AM IWTlI ID. -

Hadiotv EXPERIMENTER AUGUST -SEPTEMBER 75C
DXer's DREAM THAT ALMOST WAS SHASILAND HadioTV EXPERIMENTER AUGUST -SEPTEMBER 75c BUILD COLD QuA BREE ... a 2-FET metal moocher to end the gold drain and De Gaulle! PIUS Socket -2 -Me CB Skyhook No -Parts Slave Flash Patrol PA System IC Big Voice www.americanradiohistory.com EICO Makes It Possible Uncompromising engineering-for value does it! You save up to 50% with Eico Kits and Wired Equipment. (%1 eft ale( 7.111 e, si. a er. ortinastereo Engineering excellence, 100% capability, striking esthetics, the industry's only TOTAL PERFORMANCE STEREO at lowest cost. A Silicon Solid -State 70 -Watt Stereo Amplifier for $99.95 kit, $139.95 wired, including cabinet. Cortina 3070. A Solid -State FM Stereo Tuner for $99.95 kit. $139.95 wired, including cabinet. Cortina 3200. A 70 -Watt Solid -State FM Stereo Receiver for $169.95 kit, $259.95 wired, including cabinet. Cortina 3570. The newest excitement in kits. 100% solid-state and professional. Fun to build and use. Expandable, interconnectable. Great as "jiffy" projects and as introductions to electronics. No technical experience needed. Finest parts, pre -drilled etched printed circuit boards, step-by-step instructions. EICOGRAFT.4- Electronic Siren $4.95, Burglar Alarm $6.95, Fire Alarm $6.95, Intercom $3.95, Audio Power Amplifier $4.95, Metronome $3.95, Tremolo $8.95, Light Flasher $3.95, Electronic "Mystifier" $4.95, Photo Cell Nite Lite $4.95, Power Supply $7.95, Code Oscillator $2.50, «6 FM Wireless Mike $9.95, AM Wireless Mike $9.95, Electronic VOX $7.95, FM Radio $9.95, - AM Radio $7.95, Electronic Bongos $7.95. -

City Directory Collection
Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central Library of Rochester and Monroe County - City Directory Collection - 1944 Central -

Regional Biosecurity Plan for Micronesia and Hawaii Volume II
Regional Biosecurity Plan for Micronesia and Hawaii Volume II Prepared by: University of Guam and the Secretariat of the Pacific Community 2014 This plan was prepared in conjunction with representatives from various countries at various levels including federal/national, state/territory/commonwealth, industry, and non-governmental organizations and was generously funded and supported by the Commander, Navy Installations Command (CNIC) and Headquarters, Marine Corps. MBP PHASE 1 EXECUTIVE SUMMARY NISC Executive Summary Prepared by the National Invasive Species Council On March 7th, 2007 the U.S. Department of Navy (DoN) issued a Notice of Intent to prepare an “Environmental Impact Statement (EIS)/Overseas Environmental Impact Statement (OEIS)” for the “Relocation of U.S. Marine Corps Forces to Guam, Enhancement of Infrastructure and Logistic Capabilities, Improvement of Pier/Waterfront Infrastructure for Transient U.S. Navy Nuclear Aircraft Carrier (CVN) at Naval Base Guam, and Placement of a U.S. Army Ballistic Missile Defense (BMD) Task Force in Guam”. This relocation effort has become known as the “build-up”. In considering some of the environmental consequences of such an undertaking, it quickly became apparent that one of the primary regional concerns of such a move was the potential for unintentional movement of invasive species to new locations in the region. Guam has already suffered the eradication of many of its native species due to the introduction of brown treesnakes and many other invasive plants, animals and pathogens cause tremendous damage to its economy and marine, freshwater and terrestrial ecosystems. DoN, in consultation and concurrence with relevant federal and territorial regulatory entities, determined that there was a need to develop a biosecurity plan to address these concerns. -

INSECTICIDES from PLANTS a Review of the Literature, 1954-1971
/■■, INSECTICIDES FROM PLANTS A Review of the Literature, 1954-1971 Agriculture Handbook No. 461 >. M. r-ii cr- -•-.X €*0 ., ••> «H fTI 5:> ^':UA "X> ..; pn 1 2 Ci) :, ^'2 cr : .> oO > 5 Ç? o :í::;:'. or Agricultural Research Service UNITED STATES DEPARTMENT OF AGRICULTURE USDA, National Agricultural Library NALBldg 10301 Baltimore Blvd BeltsviHô, MD 20705-2351 Washington, D.C. Issued January 197Î For sale by the Superintendent of Documents, U.S. Government Printing Office ' Washington, D.C. 20402—Price $2 Stock Number 0100-03197 CONTENTS Page Page Cryptogams 2 Cyrillaceae 26 Agaricaceae 2 Datiscaceae 26 Dematiaceae 2 Diapensiaceae 26 Entomophthoraceae 2 Dichapetalaceae 26 Equsetaceae 2 Dioscoreaceae 26 Moniliaceae 2 Dipsacaceae___ 27 Osmundaceae 3 Dipterocarpaceae 27 Polypodiaceae 3 Ebenaceae 28 Rhodomelaceae 3 Elaeagnaceae 28 Phanerogams and spermatophytes 3 Elaeocarpaceae 28 Acanthaceae 3 Ericaceae :-. 28 Aceraceae 4 Eriocaulaceae 29 Aizoaceae 4 Erythroxylaceae 29 Alismataceae 4 Euphorbiaceae 29 Amaranthaceae 4 Fagaceae 31 Amaryllidaceae 4 Flacourtiaceae 32 Anacardiaceae 4 Gentianaceae 32 Annonaceae 6 Geraniaceae 32 Apocynaceae 7 Gesneriaceae 32 Aquifoliaceae 8 Ginkgoaceae 32 Araceae 8 Gramineae 32 Araliaceae 9 Guttiferae __. 35 Aristolochiaceae 10 Halorrhagidaceae 37 Asclepiadaceae 10 Hamamelidaceae 37 Balsaminaceae 10 Hemandiaceae 37 Begoniaceae 11 Hippocastanaceae 37 Berberidaceae 11 Humiriaceae 37 Betulaceae 11 Hypericaceae 37 Bignoniaceae 12 Icacinaceae 37 Bombacaceae 13 Juglandaceae 37 Boraginaceae 13 Labiatae 38 Burseraceae -

If O.J. LAW REV. LENYI IOBEHOST ROYALTY I ISSUES AS CAMP
ff rt THE LARGEST SURBURBAN NEWSPAPER IN THE COUNTY COMFHTELV CQVEJUX6 WOODBRUKJl, SEWAR£N, AVIMtt, 1 rout READINO, COLONIA »di MM* XXXII.—No. 83. WOODBRIDGB/N;'j . OCTOBER W. J. ohrnOutEn MasseThursdty REV. LENYI Despot Three November Holidays,ISSUES lntiewBrunswkkForWendellWillkie Teachers To Appropriate Two More Slid the thlnMerm candidate^ at- ably be provided fpr thoie who IOBEHOST Vse Ottinary Sthod Day$ For Conventions; Taxpay- AS CAMP All «o pjah to be in our party,!' cannot be accommodated in the .;. Arrangements'haVcboen made to automobiles available. ers To Pay Substitutes So Classes Can Continue finish .trans'po)rtat|on for all Who Mayor. August- P. Greincr, who If O.J. LAW do not-'hjwe,carg.,Bu8eB will prob,- was an alternate delgate fa the ROYALTY <p teachei* of, the Township convention which nominated J(r- wlfl.lje.'of.. f five,, Arijl'j a half dsjftouftij i a possible.twentypty- Schedule Of Hours Wjllkie, will ibad the Woodbridge one school'days during; the-.moiith ti i4oyembi(8P, accoifd- Township party; .He urged latt Pretender Jo Lost Throne U»I Parties Evidently 0* Calls For: C|osi«g, * \ haWood night thW /'every true friendytf irtg toa" r?pcfe^fthiittel Hf6ne(aRf6ne(ay nijrhnijhtt to^fli.to^fh>e $sarjfroard of tfe. Wshlp 'Rei»ubUcan ^ democracy,' regardless of their *iictpftl .Victor: C,;MeklRs. pending Upon Outcome . At 2 P. i JDwiir . ' expected tp turn out usual -party aflillitiftn or to thjijr flip VjHbe closed November choices lor'"any' other 'office/1 ft- Local Ptriih Monday on m«SBe.. next, ',f htoqUy .to - at-' 6 and 'U, general election day tfijrd (M National Election ; .