HOKLAS 830P List of CAB Terminated Activities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SUMMARY ACCREDITATION REPORT Nursing Services
SUMMARY ACCREDITATION REPORT Nursing Services Department, Hospital Authority Head Office Learning Programme Re-accreditation Higher Diploma in Nursing MARCH 2018 - 1 - 1. TERMS OF REFERENCE 1.1 Based on the Service Agreement (No.: VA790), the Hong Kong Council for Accreditation of Academic and Vocational Qualifications (HKCAAVQ), in the capacity of the Accreditation Authority as provided for under the Accreditation of Academic and Vocational Qualifications Ordinance (Cap 592) (hereafter Ordinance), was commissioned by the Nursing Services Department, Hospital Authority Head Office (Operator) to conduct a Learning Programme Re-accreditation Exercise with the following Terms of Reference: (a) To conduct an accreditation test as provided for in the Ordinance to determine whether the programme of the Nursing Services Department, Hospital Authority Head Office (the Operator) meets the stated objectives and QF standard and can continue to be offered as an accredited programme (i) Higher Diploma in Nursing (b) To issue to the Operator an accreditation report setting out the results of the determination in relation to (a) by HKCAAVQ. 1.2 The accreditation exercise was conducted according to the relevant accreditation guidelines referred to in the Service Agreement. The Education Bureau’s “Updated Revised Common Descriptors for Associate Degree and Higher Diploma Programmes under the New Academic Structure” was also a guiding document used by the Panel and the Operator in conducting this exercise for the Higher Diploma programme. 2. HKCAAVQ’S DETERMINATION Learning Programme Re-accreditation 2.1 HKCAAVQ has determined that the Higher Diploma in Nursing meets the stated objectives and QF standard at Level 4, and can be offered as an accredited programme with a validity period from 01 September 2018 to 30 September 2020. -
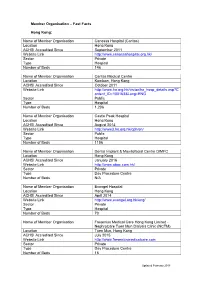
Fast Facts Hong Kong
Member Organisation – Fast Facts Hong Kong: Name of Member Organisation Canossa Hospital (Caritas) Location Hong Kong ACHSI Accredited Since September 2011 Website Link http://www.canossahospital.org.hk/ Sector Private Type Hospital Number of Beds 146 Name of Member Organisation Caritas Medical Centre Location Kowloon, Hong Kong ACHSI Accredited Since October 2011 Website Link http://www.ha.org.hk/visitor/ha_hosp_details.asp?C ontent_ID=100163&Lang=ENG Sector Public Type Hospital Number of Beds 1,206 Name of Member Organisation Castle Peak Hospital Location Hong Kong ACHSI Accredited Since August 2014 Website Link http://www3.ha.org.hk/cph/en/ Sector Public Type Hospital Number of Beds 1156 Name of Member Organisation Dental Implant & Maxillofacial Centre DIMFC Location Hong Kong ACHSI Accredited Since January 2016 Website Link http://www.aboc.com.hk/ Sector Private Type Day Procedure Centre Number of Beds N/A Name of Member Organisation Evangel Hospital Location Hong Kong ACHSI Accredited Since April 2014 Website Link http://www.evangel.org.hk/eng/ Sector Private Type Hospital Number of Beds 70 Name of Member Organisation Fresenius Medical Care Hong Kong Limited - NephroCare Tuen Mun Dialysis Clinic (NCTM) Location Tuen Mun, Hong Kong ACHSI Accredited Since July 2015 Website Link http://www.freseniusmedicalcare.com Sector Private Type Day Procedure Centre Number of Beds 15 Updated February 2018 Name of Member Organisation Fresenius Medical Care Hong Kong Limited - NephroCare Wan Chai Dialysis Clinic (NCWC) Location Wan Chai, Hong Kong -

Report of the Steering Committee on Review of Hospital Authority
Report of the Steering Committee on Review of Hospital Authority July 2015 CONTENTS Glossary .................................................................................................................. iii Executive Summary ................................................................................................ v Chapter 1 Introduction ...................................................................................... 1 Chapter 2 Work of the Steering Committee ...................................................... 6 Chapter 3 Major Challenges Facing the Hospital Authority ............................ 9 Chapter 4 Management and Organisation Structure ....................................... 13 Chapter 5 Resource Management ................................................................... 26 Chapter 6 Staff Management .......................................................................... 42 Chapter 7 Cost Effectiveness and Service Management ................................ 59 Chapter 8 Overall Management and Control .................................................. 87 Chapter 9 Conclusion ...................................................................................... 96 Annex 1 Membership of the Steering Committee on Review of Hospital Authority ....................................................................................... 102 Annex 2 Report of the Public Engagement Programme ............................. 103 Annex 3 Clustering of Hospitals and Institutions ...................................... -

Event Detail (January) 01 Jan 2019 31 Dec 20
Start End CME Points Start Date End Date Event Name Organizer Venue Event Detail Time Time (Max) (January) Caritas Medical Centre American Heart Association Advance Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Cardiovascular Life Support Provider 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (ACLS-P) Day 1 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Advance Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Cardiovascular Life Support Provider 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (ACLS-P) Day 1 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Advance Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Cardiovascular Life Support Provider 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (ACLS-P) Day 2 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Pediatric Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. Smile Pang / 01 Jan 2019 31 Dec 2019 Advanced Life Support Provider Course 08:30 17:30 Resuscitation Training Centre Wai Oi Block, 111 Wing Hong 10.00 3408 6326 / (PALS-P) Day 1 (Identical) (CMCRTC) Street, Shumshuipo, Kowloon, [email protected] Hong Kong Caritas Medical Centre American Heart Association Pediatric Caritas Medical Centre Resuscitation Training Centre, 5/F, Ms. -
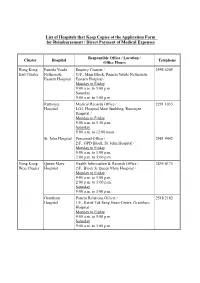
List of Hospitals That Keep Copies of the Application Form for Reimbursement / Direct Payment of Medical Expenses
List of Hospitals that Keep Copies of the Application Form for Reimbursement / Direct Payment of Medical Expenses Responsible Office / Location / Cluster Hospital Telephone Office Hours Hong Kong Pamela Youde Enquiry Counter / 2595 6205 East Cluster Nethersole G/F., Main Block, Pamela Youde Nethersole Eastern Hospital Eastern Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Ruttonjee Medical Records Office / 2291 1035 Hospital LG1, Hospital Main Building, Ruttonjee Hospital / Monday to Friday 9:00 a.m. to 5:30 p.m. Saturday 9:00 a.m. to 12:00 noon St. John Hospital Personnel Office / 2981 9442 2/F., OPD Block, St. John Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Hong Kong Queen Mary Health Information & Records Office / 2855 4175 West Cluster Hospital 2/F., Block S, Queen Mary Hospital / Monday to Friday 9:00 a.m. to 1:00 p.m. 2:00 p.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. Grantham Patient Relations Officer / 2518 2182 Hospital 1/F., Kwok Tak Seng Heart Centre, Grantham Hospital / Monday to Friday 9:00 a.m. to 5:00 p.m. Saturday 9:00 a.m. to 1:00 p.m. - 2 - Responsible Office / Location / Cluster Hospital Telephone Office Hours Kowloon Kwong Wah Medical Report Office / 3517 5216 West Cluster Hospital 1/F., Central Stack, Kwong Wah Hospital / Monday to Friday 9:00 a.m. -

Hospital Authority List of Medical Social Services Units (January 2019)
Hospital Authority List of Medical Social Services Units (January 2019) Name of Hospital Address Tel No. Fax No. 1. Alice Ho Miu Ling Nethersole 11 Chuen On Road, Tai Po, N.T. 2689 2020 2662 3152 Hospital (Non- psychiatric medical social service) 2. Bradbury Hospital 17 A Kung Kok Shan Road, 2645 8832 2762 1518 Shatin, N.T. 3. Caritas Medical Centre 111 Wing Hong Street, 3408 7709 2785 3192 Shamshuipo, Kowloon 4. Cheshire Home 128 Chung Hom Kok Road, 2899 1391 2813 8752 (Chung Hom Kok) Hong Kong 5. Cheshire Home (Shatin) 30 A Kung Kok Shan Road, 2636 7269 2636 7242 Shatin, N.T. 6. TWGHs Fung Yiu King Hospital 9 Sandy Bay Road, Hong Kong 2855 6236 2904 9021 7. Grantham Hospital 125 Wong Chuk Hang Road, 2518 2678 2580 7629 Aberdeen, Hong Kong 8. Haven of Hope Hospital 8 Haven of Hope Road, 2703 8227 2703 8230 Tseung Kwan O, Kowloon 9. Hong Kong Buddhist Hospital 10 Heng Lam Road, Lok Fu, 2339 6253 2339 6298 Kowloon 10. Kowloon Hospital Mezzanine Floor, Kowloon 3129 7806/ 3129 7838 (Ward 2B at Rehabilitation Hospital Rehabilitation Building, 3129 7831 Building ) 147A, Argyle Street, Kowloon 11. Kwong Wah Hospital 25 Waterloo Road, Kowloon 3517 2900 3517 2959 12. MacLehose Medical 7 Sha Wan Drive, 2872 7131 2872 7909 Rehabilitation Centre Pokfulam, Hong Kong Name of Hospital Address Tel No. Fax No. 13. Our Lady of Maryknoll Hospital 118 Shatin Pass Road, 2354 2285 2324 8719 Wong Tai Sin, Kowloon 14. Pok Oi Hospital Au Tau, Yuen Long, N.T. 2486 8140 2486 8095 2486 8141 15. -
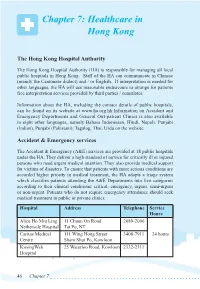
Chapter 7: Healthcare in Hong Kong
Chapter 7: Healthcare in Hong Kong The Hong Kong Hospital Authority The Hong Kong Hospital Authority (HA) is responsible for managing all local public hospitals in Hong Kong. Staff of the HA can communicate in Chinese (mainly the Cantonese dialect) and / or English. If interpretation is needed for other languages, the HA will use reasonable endeavours to arrange for patients free interpretation services provided by third parties / consulates. Information about the HA, including the contact details of public hospitals, can be found on its website at www.ha.org.hk.Information on Accident and Emergency Departments and General Out-patient Clinics is also available in eight other languages, namely Bahasa Indonesian, Hindi, Nepali, Punjabi (Indian), Punjabi (Pakistani), Tagalog, Thai, Urdu on the website. Accident & Emergency services The Accident & Emergency (A&E) services are provided at 18 public hospitals under the HA. They deliver a high standard of service for critically ill or injured persons who need urgent medical attention. They also provide medical support for victims of disasters. To ensure that patients with more serious conditions are accorded higher priority in medical treatment, the HA adopts a triage system which classifies patients attending the A&E Departments into five categories according to their clinical conditions: critical, emergency, urgent, semi-urgent or non-urgent. Patients who do not require emergency attendance should seek medical treatment in public or private clinics. Hospital Address Telephone Service Hours Alice Ho Miu Ling 11 Chuen On Road 2689-2000 Nethersole Hospital Tai Po, NT Caritas Medical 111 Wing Hong Street 3408-7911 24 hours Centre Sham Shui Po, Kowloon KwongWah 25 Waterloo Road, Kowloon 2332-2311 Hospital 46 Chapter 7 North District 9 Po Kin Road 2683-8888 Hospital Sheung Shui, NT North Lantau 1/F, 8 Chung Yan Road 3467-7000 Hospital Tung Chung, Lantau, N.T. -

List of Medical Social Services Units Under Social Welfare Department
List of Medical Social Services Units Under Social Welfare Department Hong Kong Name of Hospital/Clinic Tel. No. Email Address 1. Queen Mary Hospital 2255 3762 [email protected] 2255 3764 2. Wong Chuk Hang Hospital 2873 7201 [email protected] 3. Pamela Youde Nethersole Eastern 2595 6262 [email protected] Hospital 4. Pamela Youde Nethersole Eastern 2595 6773 [email protected] Hospital (Psychiatric Department) Kowloon Name of Hospital/Clinic Tel. No. Email Address 5. Tseung Kwan O Hospital 2208 0335 [email protected] 2208 0327 6. United Christian Hospital 3949 5178 [email protected] (Psychiatry) 7. Queen Elizabeth Hospital 3506 7021 [email protected] 3506 7027 3506 5499 3506 4021 8. Hong Kong Eye Hospital 2762 3069 [email protected] 9. Kowloon Hospital Rehabilitation 3129 7857 [email protected] Building 10. Kowloon Hospital 3129 6193 [email protected] 11. Kowloon Hospital 2768 8534 [email protected] (Psychiatric Department) 1 The New Territories Name of Hospital/Clinic Tel. No. Email Address 12. Prince of Wales Hospital 3505 2400 [email protected] 13. Shatin Hospital 3919 7521 [email protected] 14. Tai Po Hospital 2607 6304 [email protected] Sub-office Tai Po Hospital (Child and Adolescent 2689 2486 [email protected] Mental Health Centre) 15. North District Hospital 2683 7750 [email protected] 16. Tin Shui Wai Hospital 3513 5391 [email protected] 17. Castle Peak Hospital 2456 7401 [email protected] 18. Siu Lam Hospital 2456 7186 [email protected] 19. -

Hospital Authority's Planned Projects for 2021-2022
LC Paper No. CB(4)503/20-21(02) Head 708 Subhead 8083MM One-Off Grant to the Hospital Authority for Minor Works Projects 2021-22 Planned Projects Prepared by the Hospital Authority February 2021 Head 708 : Subhead 8083MM One-off Grant to the Hospital Authority for Minor Works Projects for the 2019-20 Financial Year Part A - Previously approved items and other items to commence in 2020-21 with expected expenditure in 2020-21 and/or 2021-22 Actual Approved Cumulative Revised Estimated cash flow in subsequent years Expenditure Estimate Priority / Project Expenditure Estimate Project Title (1.4.2020 to 2021-22 Post Item No. Estimate to 31.3.2020 2020-21 31.10.2020) 2022-23 2023-24 2024-25 2024-25 ($'000) (I) Previously approved items (up to 31.10.2020) with expected expenditure in 2020-21 and/or 2021-22 EMR15-604 Modernisation of lifts in Day Treatment Block and Special Block in Prince of Wales Hospital 16,794 16,540 254 254 - - - - - EMR16-104 Replacement of the local central control and monitoring system for Wong Chuk Hang Hospital 1,280 1,150 39 101 - 30 - - - EMR16-401 Replacement of fire alarm and detection system at Hospital Main Block in Tseung Kwan O 6,500 6,500 (1,371) (1,371) - - - - - Hospital EMR16-504 Replacement of 1 no. main switch board for Block A in Yan Chai Hospital 2,345 2,202 142 142 - - - - - EMR16-505 Replacement of building management system at Multi Services Complex in Yan Chai Hospital 3,500 3,148 55 55 297 - - - - EMR16-506 Replacement of the air handling unit for Department of Central Supporting Services at 1/F, 502 526 (24) (24) - - - - - Block B in Yan Chai Hospital EMR17-102 Replacement of emergency generators for Hospital Block at St. -

Caritas Medical Centre 香港九龍深水埗永康街 111 號 111 Wing Hong Street, Shamshuipo, Kowloon, Hong Kong
明愛醫院 CARITAS MEDICAL CENTRE 香港九龍深水埗永康街 111 號 111 WING HONG STREET, SHAMSHUIPO, KOWLOON, HONG KONG. TEL: 3408 7911 FAX: 2785 5755 21 December 2008 Attention News Edition: Regarding to the citizen seeking help from the Inquiry, the spokesman for Caritas Medical Centre (CMC) had the following announcement. Our Hospital Chief Executive, Dr. MA Hok Cheung, conveyed our condolence to the family and met the media at 1615 today for further clarification due to the event had made a reat concerns from the public and the media. Please see the attachment for the chronology of the events. After investigation, Dr. MA clarified that the victim had received the critical treatments including external cardiac message by a medical staff within 3 minutes and 2 times of defibrillation within 12 minutes prior the arrival of ambulance. The hospital will review the following issues and give suggestions for services improvement: 1. Review the internal protocol of the emergency handling of people in need of help inside and outside hospital compound; 2. Enhance the overall staff basic life support training; 3. Consider to purchase and place portable resuscitation equipment such as portable Automated External Defibrillator (AED) in appropriate public areas within hospital premises to facilitate the resuscitation process whenever necessary; 4. Liaise with the concerned government departments to improve the road signage for the drivers and other road users both around the hospital and within the hospital compounds. Dr. NG Fu, Chief of Service of Accident and Emergency Department, reminded the public the importance of dialing 999 for similar events with clear reporting of the location of the scene and the condition of the victim, so that the ambulance crews could bring along the appropriate resuscitation equipment to help the victim as soon as possible. -

Paper on Redevelopment of Kwong Wah Hospital Prepared By
立法會 Legislative Council LC Paper No. CB(2)836/15-16(06) Ref : CB2/PL/HS Panel on Health Services Background brief prepared by the Legislative Council Secretariat for the meeting on 15 February 2016 Redevelopment of Kwong Wah Hospital Purpose This paper summarizes the views and concerns of members of the Panel on Health Services ("the Panel") on the redevelopment of Kwong Wah Hospital ("KWH"). Background 2. Established in 1911 as part of the Tung Wah Group of Hospitals ("TWGHs"), KWH is a major acute hospital offering a comprehensive range of acute care services to population in the catchment area of the Kowloon West ("KW") Cluster1 covering Sham Shui Po, Mongkok, Wong Tai Sin2, Kwai Tsing, Tsuen Wan and North Lantau districts. The majority of the infrastructure of KWH is over 50 years old. Due to inadequate space provision and outdated building services installation, the capacity of KWH is inadequate to meet the increasing demand and requirements for healthcare services in KW Cluster. To 1 Other public hospitals in the KW Cluster include Caritas Medical Centre, Wong Tai Sin Hospital ("WTSH"), Our Lady of Maryknoll Hospital ("OLMH"), Kwai Chung Hospital, Princess Margaret Hospital and Yan Chai Hospital. 2 The Steering Committee on Review of Hospital Authority recommended, among others, in its report published in July 2015 that the Hospital Authority ("HA") should consider re-delineating Wong Tai Sin ("WTS") district from the KW Cluster to the Kowloon Central ("KC") Cluster. According to HA's Action Plan for implementation of the recommendations of the Steering Committee, KWH, WTSH and OLMH will be re-delineated from the KW Cluster to the KC Cluster to support the new KC Cluster catchment districts which will cover Kowloon City, Yau Tsim Mong and WTS districts. -

Day Surgery in Hong Kong
The Journal of One Day Surgery | 97 Featured Day Surgery Units: Day Surgery in Hong Kong JOE KM FAN, WAI LUN LAW & WAI KEI YUEN Keywords: Day surgery facilities; International practice Background Concerning surgical training in Hong Kong, there is no designated module for day surgery. The application of day Medical infrastructure in Hong Kong is mainly surgery requires careful selection of cases and special care government funded, although charity groups subsidise by specialists. In addition, the physical setup and scale of some hospitals in addition to central funding from the day surgery facilities varies greatly among the different Government. The whole territory, which has a population hospital clusters (Figures 1 & 2): some are well equipped of eight million, is served by seven hospital clusters. with operating theatres and nurse specialist for Despite the fact that some hospitals have started a scheme perioperative care; whereas some only comprise a small of “Self-Financed Items” in which patients are required to side-ward with a few beds with nurses being deployed from pay the cost of expensive drugs, such as new other surgical wards during normal working hours. chemotherapeutic agents and special instruments for some operative procedures, an average patient pays as little as US$12 per day for in-hospital charge, which includes the room as well as all investigations and treatments during the hospital stay. The remaining cost is fully covered by the Hospital Authority of the Hong Kong Special Administrative Region (HKSAR), China. Under these circumstances, there is no apparent motivation for patients to be treated as day cases, as the cost for finding a care-giver or for travelling may be much more than staying as an inpatient for several more days.