Muscle Wasting Associated with Pathologic Change Is a Risk Factor For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Triplet Repeat Length Bias and Variation in the Human Transcriptome
Triplet repeat length bias and variation in the human transcriptome Michael Mollaa,1,2, Arthur Delcherb,1, Shamil Sunyaevc, Charles Cantora,d,2, and Simon Kasifa,e aDepartment of Biomedical Engineering and dCenter for Advanced Biotechnology, Boston University, Boston, MA 02215; bCenter for Bioinformatics and Computational Biology, University of Maryland, College Park, MD 20742; cDepartment of Medicine, Division of Genetics, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115; and eCenter for Advanced Genomic Technology, Boston University, Boston, MA 02215 Contributed by Charles Cantor, July 6, 2009 (sent for review May 4, 2009) Length variation in short tandem repeats (STRs) is an important family including Huntington’s disease (10) and hereditary ataxias (11, 12). of DNA polymorphisms with numerous applications in genetics, All Huntington’s patients exhibit an expanded number of copies in medicine, forensics, and evolutionary analysis. Several major diseases the CAG tandem repeat subsequence in the N terminus of the have been associated with length variation of trinucleotide (triplet) huntingtin gene. Moreover, an increase in the repeat length is repeats including Huntington’s disease, hereditary ataxias and spi- anti-correlated to the onset age of the disease (13). Multiple other nobulbar muscular atrophy. Using the reference human genome, we diseases have also been associated with copy number variation of have catalogued all triplet repeats in genic regions. This data revealed tandem repeats (8, 14). Researchers have hypothesized that inap- a bias in noncoding DNA repeat lengths. It also enabled a survey of propriate repeat variation in coding regions could result in toxicity, repeat-length polymorphisms (RLPs) in human genomes and a com- incorrect folding, or aggregation of a protein. -

Identification of Key Genes and Pathways Involved in Response To
Deng et al. Biol Res (2018) 51:25 https://doi.org/10.1186/s40659-018-0174-7 Biological Research RESEARCH ARTICLE Open Access Identifcation of key genes and pathways involved in response to pain in goat and sheep by transcriptome sequencing Xiuling Deng1,2†, Dong Wang3†, Shenyuan Wang1, Haisheng Wang2 and Huanmin Zhou1* Abstract Purpose: This aim of this study was to investigate the key genes and pathways involved in the response to pain in goat and sheep by transcriptome sequencing. Methods: Chronic pain was induced with the injection of the complete Freund’s adjuvant (CFA) in sheep and goats. The animals were divided into four groups: CFA-treated sheep, control sheep, CFA-treated goat, and control goat groups (n 3 in each group). The dorsal root ganglions of these animals were isolated and used for the construction of a cDNA= library and transcriptome sequencing. Diferentially expressed genes (DEGs) were identifed in CFA-induced sheep and goats and gene ontology (GO) enrichment analysis was performed. Results: In total, 1748 and 2441 DEGs were identifed in CFA-treated goat and sheep, respectively. The DEGs identi- fed in CFA-treated goats, such as C-C motif chemokine ligand 27 (CCL27), glutamate receptor 2 (GRIA2), and sodium voltage-gated channel alpha subunit 3 (SCN3A), were mainly enriched in GO functions associated with N-methyl- D-aspartate (NMDA) receptor, infammatory response, and immune response. The DEGs identifed in CFA-treated sheep, such as gamma-aminobutyric acid (GABA)-related DEGs (gamma-aminobutyric acid type A receptor gamma 3 subunit [GABRG3], GABRB2, and GABRB1), SCN9A, and transient receptor potential cation channel subfamily V member 1 (TRPV1), were mainly enriched in GO functions related to neuroactive ligand-receptor interaction, NMDA receptor, and defense response. -

Tumor Suppressor and DNA Damage Response Panel 2
Tumor suppressor and DNA damage response panel 2 (55 analytes) Gene Symbol Target protein name UniProt ID (& link) Modification* *blanks mean the assay detects the ACT Actin; ACTA2 ACTA1 ACTB ACTG1 ACTC1 ACTG2 Q562L2 non‐modified peptide sequence CASC5 cancer susceptibility candidate 5 Q8NG31 pS767 CASC5 cancer susceptibility candidate 5 Q8NG31 CASP3 caspase 3, apoptosis‐related cysteine peptidase P42574 pS26 CDC25B cell division cycle 25 homolog B (S. pombe) P30305 pS16 CDC25B cell division cycle 25 homolog B (S. pombe) P30305 pS323 CDC25B cell division cycle 25 homolog B (S. pombe) P30305 CDC25C cell division cycle 25 homolog B (S. pombe) P30307 pS216 CDC25C cell division cycle 25 homolog B (S. pombe) P30307 CDCA8 cell division cycle associated 8 Q53HL2 pT16 CDK1 cyclin‐dependent kinase 1 P06493 pT161 CDK1 cyclin‐dependent kinase 1 P06493 CDK7 cyclin‐dependent kinase 7 P50613 pT17 CHEK1 CHK1 checkpoint homolog (S. pombe) O14757 pS286 CHEK2 CHK2 checkpoint homolog (S. pombe) O96017 pS379 CHEK2 CHK2 checkpoint homolog (S. pombe) O96017 pT387 CHEK2 CHK2 checkpoint homolog (S. pombe) O96017 GAPDH Glyceraldehyde‐3‐phosphate dehydrogenase P04406 LAT linker for activation of T cells O43561 pS224 LMNB1 lamin B1 P20700 pT2; pS23 LMNB1 lamin B1 P20700 pT2 LMNB1 lamin B1 P20700 pS23 MCM6 minichromosome maintenance complex component 6 Q14566 pS762 MCM6 minichromosome maintenance complex component 6 Q14566 MDM2 Mdm2, transformed 3T3 cell double minute 2, p53 binding protein (mouse) Q00987 pS166 MDM2 Mdm2, transformed 3T3 cell double minute 2, p53 binding protein (mouse) Q00987 MKI67 antigen identified by monoclonal antibody Ki‐67 P46013 pT181 MKI67 antigen identified by monoclonal antibody Ki‐67 P46013 MKI67 antigen identified by monoclonal antibody Ki‐67 P46013 pT246 MRE11A MRE11 meiotic recombination 11 homolog A (S. -

Transcriptomic Analysis of Immune Response to Bacterial Lipopolysaccharide in Zebra Finch (Taeniopygia Guttata) Cassandra S
Scalf et al. BMC Genomics (2019) 20:647 https://doi.org/10.1186/s12864-019-6016-3 RESEARCHARTICLE Open Access Transcriptomic analysis of immune response to bacterial lipopolysaccharide in zebra finch (Taeniopygia guttata) Cassandra S. Scalf1, Julia H. Chariker2, Eric C. Rouchka3 and Noah T. Ashley1* Abstract Background: Despite the convergence of rapid technological advances in genomics and the maturing field of ecoimmunology, our understanding of the genes that regulate immunity in wild populations is still nascent. Previous work to assess immune function has relied upon relatively crude measures of immunocompetence. However, with next-generation RNA-sequencing, it is now possible to create a profile of gene expression in response to an immune challenge. In this study, captive zebra finch (Taeniopygia guttata; adult males) were challenged with bacterial lipopolysaccharide (LPS) or vehicle to stimulate the innate immune system. 2 hours after injection, birds were euthanized and hypothalami, spleen, and red blood cells (RBCs) were collected. Taking advantage of the fully sequenced genome of zebra finch, total RNA was isolated, sequenced, and partially annotated in these tissue/cells. Results: In hypothalamus, there were 707 significantly upregulated transcripts, as well as 564 and 144 in the spleen and RBCs, respectively, relative to controls. Also, 155 transcripts in the hypothalamus, 606 in the spleen, and 61 in the RBCs were significantly downregulated. More specifically, a number of immunity-related transcripts (e.g., IL-1β, RSAD2, SOCS3) were upregulated among tissues/cells. Additionally, transcripts involved in metabolic processes (APOD, LRAT, RBP4) were downregulated. Conclusions: These results suggest a potential trade-off in expression of genes that regulate immunity and metabolism in birds challenged with LPS. -

SPACIA1/SAAL1 Deletion Results in a Moderate Delay in Collagen-Induced Arthritis Activity, Along with Mrna Decay of Cyclin-Dependent Kinase 6 Gene
International Journal of Molecular Sciences Article SPACIA1/SAAL1 Deletion Results in a Moderate Delay in Collagen-Induced Arthritis Activity, along with mRNA Decay of Cyclin-dependent Kinase 6 Gene Ryoji Fujii 1,*,†, Rie Komatsu 1,†, Tomoo Sato 1, Iwao Seki 2, Koji Konomi 3, Hiroyuki Aono 2, Hisateru Niki 4, Kazuo Yudoh 1, Kusuki Nishioka 5 and Toshihiro Nakajima 1,6,7 1 Institute of Medical Science, St. Marianna University School of Medicine, Kanagawa 216-8512, Japan; [email protected] (R.K.); [email protected] (T.S.); [email protected] (K.Y.); [email protected] (T.N.) 2 AYUMI Pharmaceutical Corporation, Kyoto 612-8374, Japan; [email protected] (I.S.); [email protected] (H.A.) 3 Santen Pharmaceutical Co., Ltd., Osaka 533-8651, Japan; [email protected] 4 Department of Orthopedic Surgery, St. Marianna University School of Medicine, Kanagawa 216-8511, Japan; [email protected] 5 Global Health Innovation Policy Program (GHIPP), National Graduate Institute for Policy Studies (GRIPS), Tokyo 106-8677, Japan; [email protected] 6 Institute of Medical Science, Tokyo Medical University, Tokyo 160-8402, Japan 7 Misato Marine Hospital, Kochi 781-0112, Japan * Correspondence: [email protected]; Tel.: +81-44-977-8111 (ext. 4213) † These authors contributed equally to this work. Received: 31 October 2018; Accepted: 29 November 2018; Published: 30 November 2018 Abstract: This study was performed to elucidate the molecular function of the synoviocyte proliferation-associated in collagen-induced arthritis (CIA) 1/serum amyloid A-like 1 (SPACIA1/SAAL1) in mice CIA, an animal model of rheumatoid arthritis (RA), and human RA-synovial fibroblasts (RASFs). -

Engineered Type 1 Regulatory T Cells Designed for Clinical Use Kill Primary
ARTICLE Acute Myeloid Leukemia Engineered type 1 regulatory T cells designed Ferrata Storti Foundation for clinical use kill primary pediatric acute myeloid leukemia cells Brandon Cieniewicz,1* Molly Javier Uyeda,1,2* Ping (Pauline) Chen,1 Ece Canan Sayitoglu,1 Jeffrey Mao-Hwa Liu,1 Grazia Andolfi,3 Katharine Greenthal,1 Alice Bertaina,1,4 Silvia Gregori,3 Rosa Bacchetta,1,4 Norman James Lacayo,1 Alma-Martina Cepika1,4# and Maria Grazia Roncarolo1,2,4# Haematologica 2021 Volume 106(10):2588-2597 1Department of Pediatrics, Division of Stem Cell Transplantation and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 2Stanford Institute for Stem Cell Biology and Regenerative Medicine, Stanford School of Medicine, Stanford, CA, USA; 3San Raffaele Telethon Institute for Gene Therapy, Milan, Italy and 4Center for Definitive and Curative Medicine, Stanford School of Medicine, Stanford, CA, USA *BC and MJU contributed equally as co-first authors #AMC and MGR contributed equally as co-senior authors ABSTRACT ype 1 regulatory (Tr1) T cells induced by enforced expression of interleukin-10 (LV-10) are being developed as a novel treatment for Tchemotherapy-resistant myeloid leukemias. In vivo, LV-10 cells do not cause graft-versus-host disease while mediating graft-versus-leukemia effect against adult acute myeloid leukemia (AML). Since pediatric AML (pAML) and adult AML are different on a genetic and epigenetic level, we investigate herein whether LV-10 cells also efficiently kill pAML cells. We show that the majority of primary pAML are killed by LV-10 cells, with different levels of sensitivity to killing. Transcriptionally, pAML sensitive to LV-10 killing expressed a myeloid maturation signature. -

Muscle Progenitor Specification and Myogenic Differentiation
ARTICLE https://doi.org/10.1038/s41467-020-19999-w OPEN Muscle progenitor specification and myogenic differentiation are associated with changes in chromatin topology Nan Zhang1, Julen Mendieta-Esteban 2, Alessandro Magli 3,4, Karin C. Lilja1, Rita C. R. Perlingeiro 3,4, ✉ Marc A. Marti-Renom 2,5,6,7, Aristotelis Tsirigos 1 & Brian David Dynlacht1 1234567890():,; Using Hi-C, promoter-capture Hi-C (pCHi-C), and other genome-wide approaches in skeletal muscle progenitors that inducibly express a master transcription factor, Pax7, we system- atically characterize at high-resolution the spatio-temporal re-organization of compartments and promoter-anchored interactions as a consequence of myogenic commitment and dif- ferentiation. We identify key promoter-enhancer interaction motifs, namely, cliques and networks, and interactions that are dependent on Pax7 binding. Remarkably, Pax7 binds to a majority of super-enhancers, and together with a cadre of interacting transcription factors, assembles feed-forward regulatory loops. During differentiation, epigenetic memory and persistent looping are maintained at a subset of Pax7 enhancers in the absence of Pax7. We also identify and functionally validate a previously uncharacterized Pax7-bound enhancer hub that regulates the essential myosin heavy chain cluster during skeletal muscle cell differ- entiation. Our studies lay the groundwork for understanding the role of Pax7 in orchestrating changes in the three-dimensional chromatin conformation in muscle progenitors. 1 Department of Pathology and Perlmutter Cancer Institute, New York University School of Medicine, New York, NY 10016, USA. 2 CNAG-CRG, Centre for Genomic Regulation (CRG), Barcelona Institute of Science and Technology (BIST), Barcelona, Spain. 3 Department of Medicine, Lillehei Heart Institute, University of Minnesota, Minneapolis, MN 55455, USA. -

PRODUCT SPECIFICATION Prest Antigen SAAL1 Product Datasheet
PrEST Antigen SAAL1 Product Datasheet PrEST Antigen PRODUCT SPECIFICATION Product Name PrEST Antigen SAAL1 Product Number APrEST80690 Gene Description serum amyloid A-like 1 Alternative Gene FLJ41463 Names Corresponding Anti-SAAL1 (HPA039004) Antibodies Description Recombinant protein fragment of Human SAAL1 Amino Acid Sequence Recombinant Protein Epitope Signature Tag (PrEST) antigen sequence: IGSTVYSKHWLFGVLSGLIQIVSPENTKSSSDDEEQLTELDEEMENEICR VWDMSMDEDVALFLQEFNAPDIFMGVLAKSKCPRLR Fusion Tag N-terminal His6ABP (ABP = Albumin Binding Protein derived from Streptococcal Protein G) Expression Host E. coli Purification IMAC purification Predicted MW 27 kDa including tags Usage Suitable as control in WB and preadsorption assays using indicated corresponding antibodies. Purity >80% by SDS-PAGE and Coomassie blue staining Buffer PBS and 1M Urea, pH 7.4. Unit Size 100 µl Concentration Lot dependent Storage Upon delivery store at -20°C. Avoid repeated freeze/thaw cycles. Notes Gently mix before use. Optimal concentrations and conditions for each application should be determined by the user. Product of Sweden. For research use only. Not intended for pharmaceutical development, diagnostic, therapeutic or any in vivo use. No products from Atlas Antibodies may be resold, modified for resale or used to manufacture commercial products without prior written approval from Atlas Antibodies AB. Warranty: The products supplied by Atlas Antibodies are warranted to meet stated product specifications and to conform to label descriptions when used and stored properly. Unless otherwise stated, this warranty is limited to one year from date of sales for products used, handled and stored according to Atlas Antibodies AB's instructions. Atlas Antibodies AB's sole liability is limited to replacement of the product or refund of the purchase price. -

WO 2012/142330 Al 18 October 2012 (18.10.2012) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2012/142330 Al 18 October 2012 (18.10.2012) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12Q 1/68 (2006.01) A61P 35/00 (2006.01) kind of national protection available): AE, AG, AL, AM, G0 33/574 (2006.01) A61P 35/04 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, PCT/US2012/033386 HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 12 April 2012 (12.04.2012) MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/476,056 15 April 201 1 (15.04.201 1) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (71) Applicants (for all designated States except US): UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, BROWN UNIVERSITY [US/US]; 1 Prospect Street, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, Providence, RI 02912 (US). -
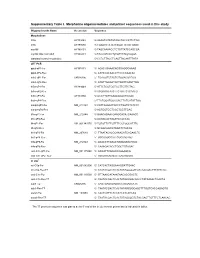
Supplementary Table I. Morpholino Oligonucleotides and Primer Sequences Used in This Study
Supplementary Table I. Morpholino oligonucleotides and primer sequences used in this study Oligonucleotide Name Accession Sequence Morpholinos tlr5a AY389449 5'-AAAGTGTATGTAGCTGCCATTCTGG tlr5b AY389450 5'-TGAATGTATATCCCATTCTGTGAGC myd88 AY388401 5'-TAGCAAAACCTCTGTTATCCAGCGA myd88 5bp mismatch AY388401 5'-TAcCAtAACCTgTGTTATCgAGgGA standard control morpholino 5'-CCTCTTACCTCAGTTACAATTTATA qRT-PCR ppial-qP1-Fw AY391451 5’- ACACTGAAACACGGAGGCAAAG ppial-qP2-Rev 5’- CATCCACAACCTTCCCGAACAC irak3-qP1-Fw CK026195 5’- TGAGGTCTACTGTGGACGATGG irak3-qP2-Rev 5’- ATGTTAGGATGCTGGTTGAGTTGG tlr5a-qP1-Fw AY389449 5’-ATTCTGGTGGTGCTTGTTGTAG tlr5a-qP2-Rev 5’-ACGAGGTAACTTCTGTTCTCAATG tlr5b-qP3-Fw AY389450 5’-GCGTTGTTGAAGAGGCTGGAC tlr5b-qP4-Rev 5’-TTCTGGATGGCCACTTCTCATATTGG mmp9-qP3-Fw NM_213123 5’-CATTAAAGATGCCCTGATGTATCCC mmp9-qP4-Rev 5’-AGTGGTGGTCCGTGGTTGAG il1b-qP1-Fw NM_212844 5’-GAACAGAATGAAGCACATCAAACC il1b-qP2-Rev 5’-ACGGCACTGAATCCACCAC il8-qP1-Fw XM_001342570 5’-TGTGTTATTGTTTTCCTGGCATTTC il8-qP2-Rev 5’-GCGACAGCGTGGATCTACAG ifn1-qP3-Fw NM_207640 5’- TTAATACACGCAAAGATGAGAACTC ifn1-qP4-Rev 5’- GCCAAGCCATTCGCAAGTAG tnfa-qP5-Fw NM_212829 5’- AGACCTTAGACTGGAGAGATGAC tnfa-qP6-Rev 5’- CAAAGACACCTGGCTGTAGAC cxcl-C1c-qP1-Fw NM_001115060 5’- GGCATTCACACCCAAAGCG cxcl-C1c-qP2_Rev 5’- GCGAGCACGATTCACGAGAG * In situ ccl-C5a-Fw NM_001082906 5’- CATCACTAGGAAAGGATTGAAC ccl-C5a-Rev-T7 5’- TAATACGACTCACTATAGGGGATGTCAAAGACTTTATTCAC cxcl-C1c-Fw NM_001115060 5’- GTTAAACATAAATAACACCGACTC cxcl-C1c-Rev-T7 5’- TAATACGACTCACTATAGGGACACCCTATAAAACTGAGTA irak3-Fw CK026195 5’- CAGTGAGAGAGGCATGAAACATC -

Fatty Acid Metabolism Driven Mitochondrial Bioenergetics Promotes Advanced Developmental Phenotypes in Human Induced Pluripotent Stem Cell Derived Cardiomyocytes
Fatty acid metabolism driven mitochondrial bioenergetics promotes advanced developmental phenotypes in human induced pluripotent stem cell derived cardiomyocytes Chrishan J.A. Ramachandra1,a,b, Ashish Mehta1,c, Philip Wonga,b,d,e*, K.P. Myu Mai Jaa, Regina Fritsche-Danielsonf, Ratan V. Bhatg, Derek J. Hausenloya,b,h,i,j, Jean-Paul Kovalikb and Winston Shima,b,k* aNational Heart Research Institute Singapore, National Heart Centre Singapore bCardiovascular & Metabolic Disorders Program, Duke-NUS Medical School, Singapore cPSC and Phenotyping Laboratory, Victor Chang Cardiac Research Institute, Sydney, Australia dDepartment of Cardiology, National Heart Centre Singapore eSchool of Materials Science and Engineering, Nanyang Technological University, Singapore fCardiovascular and Metabolic Disease Innovative Medicines and Early Development Unit, AstraZeneca Research and Development, Gothenburg, Sweden gStrategy and External Innovation Department, AstraZeneca, Gothenburg, Sweden hThe Hatter Cardiovascular Institute, University College London, United Kingdom iBarts Heart Centre, St Barthlomew’s Hospital, London, United Kingdom jYong Loo Lin School of Medicine, National University of Singapore kHealth and Social Sciences Cluster, Singapore Institute of Technology 1Both authors contributed equally Running title: Cardiomyocyte metabolism and bioenergetics *Corresponding authors: Philip Wong National Heart Centre Singapore, 5 Hospital Drive, Singapore 169609 Email: [email protected]; Phone: +65 6704 8964; Fax: +65 6844 9053 Winston Shim