Exploring the Benefits and Challenges of Establishing a DRI-Like Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guideline for Dietary Fibre Intake
Guideline for dietary fibre intake Gezondheidsraad Health Council of the Netherlands To the Minister of Health, Welfare and Sport Subject : Presentation of advisory report Guideline for dietary fibre intake Your reference:- Our reference : U 383/CS/cn/754-C Enclosures : 1 Date : March 21, 2006 Dear Minister, I hereby present an advisory report concerning the Guideline for Dietary Fibre Intake, which has been prepared, at the request of my predecessor Professor JGAJ Hautvast, by the Health Council's Committee on Dietary Fibre and reviewed by the Standing Committee on Nutrition and the Standing Committee on Medicine. I have today also presented this report to the Minister of Agriculture, Nature and Food Quality. This is the fourth in a series of advisory reports designed to revise the Dutch dietary ref- erence intakes (Nederlandse Voedingsnormen), which were adopted in 1992 by the former Food and Nutrition Council (Voedingsraad). Dietary fibre received very little attention in that report, however. The advisory report Guidelines for a Healthy Diet (Richtlijnen Goede Voeding), which was published by the Food and Nutrition Council in 1986 and is currently being revised, also included a very brief passage about dietary fibre. The attached advisory report is therefore the first thorough evaluation of the physiological effects of dietary fibre to have been undertaken in the Netherlands. The Committee has chosen not to set a dietary reference intake for fibre, but instead to issue a guideline. This decision is motivated by the fact that dietary fibre is the collective term for a group of substances with very wide-ranging physiological effects. -

Nutrition Guideline: Vitamins and Minerals
Nutrition Guideline For Professional Reference Only Vitamins and Minerals Applicable to: Nurses, Physicians and Other Health Professionals Recommendations Most individuals can meet their vitamin and mineral needs by healthy eating using Canada’s Food Guide. The following strategies can help an individual achieve appropriate intakes of vitamins and minerals: • Choosing a variety of foods from all four food groups of Canada’s Food Guide every day and eating the recommended number of servings from each food group every day. • Choosing meals that include at least three of the four food groups. • For adults: Eating three regular meals, and snacks if needed, throughout the day. • For children: Providing three regular meals and two to three snacks every day. Provide snacks that include foods from Canada’s Food Guide. • Considering taking a vitamin or mineral supplement if you are not meeting the recommended number of servings of each food group from Canada’s Food Guide. Ask for advice from your physician or health care provider. The following people need to take vitamin or mineral supplements: o Women who are pregnant, breastfeeding, or who could become pregnant, need a multivitamin containing folic acid and vitamin B12 every day. o Pregnant women need to ensure that their multivitamin contains iron. o Refer to the guideline Calcium and Vitamin D for recommendations about vitamin D supplementation • Recognizing that single-nutrient supplements carry a higher risk of adverse reactions. Seek referral to a physician or Registered Dietitian if you are considering taking one. • Notifying your physician or Registered Dietitian if you take a multivitamin-mineral, herbal or any other type of nutrition supplement. -

DRIDIETARY REFERENCE INTAKES Thiamin, Riboflavin, Niacin, Vitamin
Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline http://www.nap.edu/catalog/6015.html DIETARY REFERENCE INTAKES DRI FOR Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline A Report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients Food and Nutrition Board Institute of Medicine NATIONAL ACADEMY PRESS Washington, D.C. Copyright © National Academy of Sciences. All rights reserved. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline http://www.nap.edu/catalog/6015.html NATIONAL ACADEMY PRESS • 2101 Constitution Avenue, N.W. • Washington, DC 20418 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This project was funded by the U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion, Contract No. 282-96-0033, T01; the National Institutes of Health Office of Nutrition Supplements, Contract No. N01-OD-4-2139, T024, the Centers for Disease Control and Prevention, National Center for Chronic Disease Preven- tion and Health Promotion, Division of Nutrition and Physical Activity; Health Canada; the Institute of Medicine; and the Dietary Reference Intakes Corporate Donors’ Fund. -

Dietary Reference Intakes for Japanese (2015) Ministry of Health
Dietary Reference Intakes for Japanese (2015) Ministry of Health, Labour and Welfare Health Service Bureau, Ministry of Health, Labour and Welfare, JAPAN 1-2-2 Kasumigaseki, Chiyoda-ku, Tokyo, Japan 100-8916 March 2018 This translation work was realized with the great help of Dr. Satoshi SASAKI (Toyko University) and Dr. Aki SAITO (National Institutes of Biomedical Innovation, Health and Nutrition). Contents I Development and Application of Dietary Reference Intakes for Japanese 1 1 Introduction 2 2 Basics of Development 6 3 Considerations for Development 13 4 Application of the Dietary Reference Intakes 19 II Energy and Nutrients 32 Energy 33 Protein 66 Dietary Fat 81 Carbohydrate 104 Energy Providing Nutrients’ Balance 115 Vitamins (1) Fat-soluble Vitamins (2) Water-soluble Vitamins Vitamin A 126 Vitamin B1 154 Vitamin D 131 Vitamin B2 157 Vitamin E 137 Niacin 160 Vitamin K 140 Vitamin B6 163 Vitamin B12 167 Folate 170 Pantothenic acid 174 Biotin 177 Vitamin C 180 Minerals (1) Macrominerals (2) Microminerals Sodium 201 Iron 236 Potassium 206 Zinc 246 Calcium 210 Copper 249 Magnesium 216 Manganese 251 Phosphorus 218 Iodine 253 Selenium 257 Chromium 260 Molybdenum 262 I Development and Application I Development and Application of Dietary Reference Intakes for Japanese 1 I Development and Application 1.Introduction The Dietary Reference Intakes for Japanese proposes reference values for the intake of energy and nutrients, in the Japanese population, comprising both healthy individuals and groups, for the promotion and maintenance of health, and to prevent the occurrence of lifestyle- related diseases (LRDs). The objectives behind the development of the Dietary Reference Intakes for Japanese (2015) are shown in Figure 1. -

Vitamin B12 Among Vegetarians: Status, Assessment and Supplementation
nutrients Review Vitamin B12 among Vegetarians: Status, Assessment and Supplementation Gianluca Rizzo 1, Antonio Simone Laganà 2,*, Agnese Maria Chiara Rapisarda 3, Gioacchina Maria Grazia La Ferrera 4, Massimo Buscema 5, Paola Rossetti 5, Angela Nigro 5, Vincenzo Muscia 5, Gaetano Valenti 3, Fabrizio Sapia 3, Giuseppe Sarpietro 3, Micol Zigarelli 3 and Salvatore Giovanni Vitale 2 1 Vico Sant’andrea 5, Ritiro, Messina 98152, Italy; [email protected] 2 Unit of Gynecology and Obstetrics, Department of Human Pathology in Adulthood and Childhood, “G. Barresi”, University of Messina, Via Consolare Valeria 1, Messina 98125, Italy; [email protected] 3 Department of General Surgery and Medical Surgical Specialties, University of Catania, Via S. Sofia 78, Catania 95124, Italy; [email protected] (A.M.C.R.); [email protected] (G.V.); [email protected] (F.S.); [email protected] (G.S.); [email protected] (M.Z.) 4 Department of Gastroenterology and Digestive Endoscopy Maddalena Raimondi San Cataldo, Via Forlanini 5, San Cataldo, Caltanissetta 93017, Italy; [email protected] 5 Unit of Diabetology and Endocrino-Metabolic Diseases, Hospital for Emergency Cannizzaro, Via Messina 829, Catania 95126, Italy; [email protected] (M.B.); [email protected] (P.R.); [email protected] (A.N.); [email protected] (V.M.) * Correspondence: [email protected]; Tel.: +39-090-221-2183; Fax: +39-090-293-7083 Received: 3 September 2016; Accepted: 23 November 2016; Published: 29 November 2016 Abstract: Cobalamin is an essential molecule for humans. It acts as a cofactor in one-carbon transfers through methylation and molecular rearrangement. These functions take place in fatty acid, amino acid and nucleic acid metabolic pathways. -

Choline an Essential Nutrient for Public Health
Continuing Education Elevating Awareness and Intake of Choline An Essential Nutrient for Public Health Marie Caudill, PhD, RD Steven Zeisel, MD, PhD Kerry-Ann da Costa, PhD Betsy Hornick, MS, RD Emerging science has revealed that choline plays important Choline is the newest roles in health throughout the life cycle with potentially essential nutrient. serious health consequences associated with inadequate choline intakes. Recent national consumption data indicate that the vast majority of Americans are falling short of recommended intakes. Increased education, including In 2009, the Choline Science Summit was held in Washington, District of Columbia, bringing together recommendations to consume more choline-rich foods, prominent scientists and researchers along with nutrition is needed to improve choline intakes for optimal health. leaders from organizations including the American Nutr Today. 2011;46(5):235–241 College of Obstetricians and Gynecologists, American Dietetic Association, American Society for Nutrition, International Life Sciences Institute, National WIC Association, and US Department of Agriculture (USDA). holine was discovered as a vitamin in 1862, The symposium addressed the latest science on choline but it was more than a century later that it and gathered insights for helping raise awareness Cwas recognized as an essential nutrient. It was and intake of this essential nutrient, especially among officially recognized as an essential nutrient by the vulnerable populations. It included discussions of Institute of Medicine (IOM) in 1998, and an adequate choline’s critical role in human health and development intake (AI) was established based on estimated throughout the life cycle, the role of genetics in dietary intakes and studies reporting liver damage determining choline requirements, and a closer look with lower choline intakes.1 Choline is essential for at the gaps in choline requirements compared with liver and brain function, lipid metabolism, and for actual intakes. -

Human Vitamin and Mineral Requirements
Human Vitamin and Mineral Requirements Report of a joint FAO/WHO expert consultation Bangkok, Thailand Food and Agriculture Organization of the United Nations World Health Organization Food and Nutrition Division FAO Rome The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concern- ing the delimitation of its frontiers or boundaries. All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to the Chief, Publishing and Multimedia Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] © FAO 2001 FAO/WHO expert consultation on human vitamin and mineral requirements iii Foreword he report of this joint FAO/WHO expert consultation on human vitamin and mineral requirements has been long in coming. The consultation was held in Bangkok in TSeptember 1998, and much of the delay in the publication of the report has been due to controversy related to final agreement about the recommendations for some of the micronutrients. A priori one would not anticipate that an evidence based process and a topic such as this is likely to be controversial. -

Nutraceuticals and Food Supplements Sector in Japan
1 CONTENTS 4.4.4.3. Basic and Processed Healthy Beverages 4.5. Import Trends I. NUTRACEUTICALS: SCALE AND ECONOMIC SIGNIFICANCE 4.5.1. Import Trends for Basic and Processed Food 1.1. Definition.Potential health benefits of nutraceuticals 4.5.2. Import Trends in the Health Ingredients and Dietary Supplements Sector II. GLOBAL NUTRACEUTICAL MARKET OVERVIEW 4.5.3. Import Trends in the Organic Sector 2.1 Nutraceutical Industry 4.5.4. Import Trends from Switzerland 2.2. European market size 5. DISTRIBUTION CHANNELS 2.3. Growth of Asia-Pacific Marketplace 5.1. Distribution of Food and Beverage Products: Overview III. NUTRACEUTICALS GLOBAL MARKET, 5.1.1. Distribution of Health Food and Beverages 3.1 Nutraceutical ingredients market segmentation 5.1.2. Distribution of Organic Products 3.1.1.Functional food 5.2. Imported Food and Beverage Sector: Overview 3.1.2.Phytonutrients 5.2.1. Retail of Imported Packaged Food and Beverages 3.1.3 Probiotics and prebiotics 5.2.2. Import and Distribution of Functional Ingredients and Supplements 3.2.Demand for new ingredients &product innovation 5.2.2.1. Bulk Supply of Ingredients 3.3Nutraceutical sector breakdown 5.2.2.2. Commissioned Manufacturing 4. ECONOMIC DRIVERS AND FUTURE TRENDS 6. PRICING 4.1. POPULAR SALES CHANNELS AND CONSUMER ATTITUDES 7. CONSUMER TRENDS 5. REAL VERSUS PERCEIVED BENEFITS OF FUNCTIONAL FOODS AND 7.1. Consumer General Profile Regarding Food VMS PRODUCTS 7.2. Consumer Key Segmentation 6. DISTRIBUTION CHANELS: DIGITAL & MOBILE MARKETING: 7.3. Household Expenditures ENABLING PERSONALISED CUSTOMER SERVICE 7.4. Evolution of Consumption and Upcoming Trends in the Health and 7. -

Dietary Reference Intakes for Water, Potassium, Sodium, Chloride
DIETARY REFERENCE INTAKES DRI FOR Water, Potassium, Sodium, Chloride, and Sulfate Panel on Dietary Reference Intakes for Electrolytes and Water Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board THE NATIONAL ACADEMIES PRESS 500 Fifth Street, N.W. Washington, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Institute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This study was supported by a contract between the National Academy of Sciences and the U.S. Department of Health and Human Services’ Office of Disease Prevention and Health Promotion, Contract No. 282-96-0033, T03; the National Heart, Lung, and Blood Institute of the National Institutes of Health; the U.S. Environmental Protection Agency; the U.S. De- partment of Agriculture; Health Canada; the Institute of Medicine; the Dietary Reference Intakes Private Foundation Fund—International Life Sciences Institute-North America and the Dannon Institute; and the Dietary Reference Intakes Corporate Donors’ Fund. Contribu- tors to the Fund have included Roche Vitamins, M&M/Mars, Mead Johnson Nutritionals, and the Nabisco Foods Group. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the organizations or agencies that provided support for the project. Library of Congress Cataloging-in-Publication Data Institute of Medicine (U.S.). -

Folate Food Source, Usual Intake, and Folate Status in Korean Adults
Nutrition Research and Practice 2018;12(1):47-51 ⓒ2018 The Korean Nutrition Society and the Korean Society of Community Nutrition http://e-nrp.org Folate food source, usual intake, and folate status in Korean adults Young-Nam Kim1 and Youn-Ok Cho2§ 1Department of Food and Nutrition, Songwon University, Gwangju 61756, Korea 2Department of Food and Nutrition, Duksung Women’s University, 33 Samyangro, 114 Gill, Dobonggu, Seoul 01369, Korea BACKGROUND/OBJECTTIVES: The purposes of the study were to investigate folate intakes and plasma folate concentrations as well as estimate folate status in Korean healthy adults. SUBJECTS/METHODS: A total of 254 healthy 19- to 64-year-old adults (68 men and 186 women) living in Seoul metropolitan area, Gumi, and Kwangju, Korea participated. Three consecutive 24-hour dietary recalls, information on folate supplementation, and fasting blood samples were collected from the subjects. RESULTS: The mean dietary folate intakes were 587.4 and 499.2 μg dietary folate equivalent (DFE)/day for men and women, respectively. The median dietary intakes of men and women were 566.6 and 474.6 μg DFE/day, respectively. Forty subjects (16.7% of total) less total folate than the estimated average requirement (EAR). Folate intakes of 23.3% of men and 34.8% of women aged 19-29 years did not meet the EAR for folate. Major food sources consumed for dietary folate were baechukimchi (Chinese cabbage kimchi), rice, spinach, eggs, and laver, which provided 44% of dietary folate intake for the subjects. Plasma folate concentrations were 23.4 nmol/L for men and 28.3 nmol/L for women, and this level was significantly lower in men than in women. -
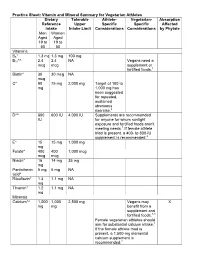
Practice Sheet: Vitamin and Mineral Summary for Vegetarian Athletes Dietary Reference Intake Tolerable Upper Intake Limit Athlet
Practice Sheet: Vitamin and Mineral Summary for Vegetarian Athletes Dietary Tolerable Athlete- Vegetarian- Absorption Reference Upper Specific Specific Affected Intake Intake Limit Considerations Considerations by Phytate Men Women Aged Aged 19 to 19 to 50 50 Vitamins B6* 1.3 mg 1.3 mg 100 mg B12*^ 2.4 2.4 NA Vegans need a mcg mcg supplement or fortified foods.1 Biotin* 30 30 mcg NA mcg C* 90 75 mg 2,000 mg Target of 100 to mg 1,000 mg has been suggested for repeated, sustained strenuous exercise.2 D*^ 600 600 IU 4,000 IU Supplements are recommended IU for anyone for whom sunlight exposure and fortified foods aren't meeting needs.1 If female athlete triad is present, a 400- to 800-IU supplement is recommended.2 E* 15 15 mg 1,000 mg mg Folate* 400 400 1,000 mcg mcg mcg Niacin* 16 14 mg 35 mg mg Pantothenic 5 mg 5 mg NA acid* Riboflavin* 1.3 1.1 mg NA mg Thiamin* 1.2 1.1 mg NA mg Minerals Calcium*^ 1,000 1,000 2,500 mg Vegans may X mg mg benefit from a supplement and fortified foods.1,3 Female vegetarian athletes should aim for substantial calcium intake.1 If the female athlete triad is present, a 1,500-mg elemental calcium supplement is recommended.2 Iodine^ 150 150 1,100 mcg Some vegans mcg mcg may not get enough without consuming iodized salt or sea vegetables.1 Iron*^ 8 mg 8 to 18 45 mg Increased need Increased need X mg for athletes by a for vegetarians factor of 1.3 to by a factor of 1.7.4 1.8.4 Female vegetarian athletes should aim for substantial iron intake.1 Magnesium* 400 310 to 350 mg (for to 320 mg supplemental 420 magnesium) mg Selenium* 55 55 mcg 400 mcg mcg Zinc*^ 11 8 mg 40 mg Possible that X mg needs are higher, especially for vegans, by factor of 1.5 to 2.5 *Micronutrients of concern for athletes2 ^Micronutrients of concern for vegetarians1 — Source: Institute of Medicine Dietary Reference Intakes Additional Considerations • The primary goal for athletes is to reach at least the Recommended Dietary Allowance for vitamins and minerals. -

Mypyramid Food Intake Pattern Modeling for the Dietary Guidelines Advisory Committee
REPORT MyPyramid Food Intake Pattern Modeling for the Dietary Guidelines Advisory Committee Patricia Britten, PhD1; Joan Lyon, MS, RD1; Connie M. Weaver, PhD2; Penny M. Kris-Etherton, PhD3; Theresa A. Nicklas, DrPH4; Jennifer A. Weber, MPH, RD5; Carole A. Davis, MS, RD1 ABSTRACT Modeling analyses using the MyPyramid intake patterns were conducted in collaboration with the 2005 Dietary Guidelines Advisory Committee in response to their research questions and to determine likely effects of possible recommendations on overall dietary adequacy. Scenarios modeled included the feasibility of using the food patterns for lacto-ovo-vegetarian diets, of varying fat levels within the patterns, and of increasing dietary flexibility through food group substitutions. Food pattern modeling was a useful tool to identify possible impacts on diet quality of potential Dietary Guidelines recommendations. Modeling analyses can help researchers explore the overall effect of specific dietary recommendations on intake patterns. Key Words: MyPyramid, Dietary Guidelines for Americans, food intake patterns, dietary guidance (J Nutr Educ Behav. 2006;38:S143-S152) INTRODUCTION among the Dietary Guidelines Advisory Committee (DGAC) as a whole, individual subcommittees, and federal In January 2005, the U.S. Departments of Agriculture staff from USDA and HHS. Several collaborative sessions (USDA) and Health and Human Services (HHS) jointly took place during the DGAC’s 5 formal meetings, which released the Dietary Guidelines for Americans (Dietary were open to the public. Table 1 shows a brief side-by-side Guidelines), which form the basis of federal food and nu- timeline of the 2 development processes and key points of trition policy. Shortly thereafter, USDA released the My- collaboration.