5 -Triphosphate RNA Requires Base-Paired Structures to Activate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Novel 65-Bp Indel in the GOLGB1 Gene Is Associated with Chicken Growth and Carcass Traits
animals Article A Novel 65-bp Indel in the GOLGB1 Gene Is Associated with Chicken Growth and Carcass Traits Rong Fu 1,2, Tuanhui Ren 1,2, Wangyu Li 1,2, Jiaying Liang 1,2, Guodong Mo 1,2, Wen Luo 1,2, Danlin He 1,2, Shaodong Liang 1,2 and Xiquan Zhang 1,2,* 1 Department of Animal Genetics, Breeding and Reproduction, College of Animal Science, South China Agricultural University, Guangzhou 510642, China; [email protected] (R.F.); [email protected] (T.R.); [email protected] (W.L.); [email protected] (J.L.); [email protected] (G.M.); [email protected] (W.L.); [email protected] (D.H.); [email protected] (S.L.) 2 Guangdong Provincial Key Lab of Agro-Animal Genomics and Molecular Breeding, and Key Laboratory of Chicken Genetics, Breeding and Reproduction, Ministry of Agriculture, Guangzhou 510642, China * Correspondence: [email protected] Received: 20 February 2020; Accepted: 4 March 2020; Published: 12 March 2020 Simple Summary: Many Chinese-local chickens show slow-growing and low-producing performance, which is not conductive to the development of the poultry industry. The identification of thousands of indels in the last twenty years has helped us to make progress in animal genetics and breeding. Golgin subfamily B member 1 (GOLGB1) is located on chromosome 1 in chickens. Previous study showed that a large number of QTLs on the chicken chromosome 1 were related to the important economic traits. However, the biological function of GOLGB1 gene in chickens is still unclear. In this study, we detected a novel 65-bp indel in the fifth intron of the chicken GOLGB1 gene. -
![Viewed Previously [4]](https://docslib.b-cdn.net/cover/6213/viewed-previously-4-126213.webp)
Viewed Previously [4]
Barlow et al. BMC Biology (2018) 16:27 https://doi.org/10.1186/s12915-018-0492-9 RESEARCHARTICLE Open Access A sophisticated, differentiated Golgi in the ancestor of eukaryotes Lael D. Barlow1, Eva Nývltová2,3, Maria Aguilar1, Jan Tachezy2 and Joel B. Dacks1,4* Abstract Background: The Golgi apparatus is a central meeting point for the endocytic and exocytic systems in eukaryotic cells, and the organelle’s dysfunction results in human disease. Its characteristic morphology of multiple differentiated compartments organized into stacked flattened cisternae is one of the most recognizable features of modern eukaryotic cells, and yet how this is maintained is not well understood. The Golgi is also an ancient aspect of eukaryotes, but the extent and nature of its complexity in the ancestor of eukaryotes is unclear. Various proteins have roles in organizing the Golgi, chief among them being the golgins. Results: We address Golgi evolution by analyzing genome sequences from organisms which have lost stacked cisternae as a feature of their Golgi and those that have not. Using genomics and immunomicroscopy, we first identify Golgi in the anaerobic amoeba Mastigamoeba balamuthi. We then searched 87 genomes spanning eukaryotic diversity for presence of the most prominent proteins implicated in Golgi structure, focusing on golgins. We show some candidates as animal specific and others as ancestral to eukaryotes. Conclusions: None of the proteins examined show a phyletic distribution that correlates with the morphology of stacked cisternae, suggesting the possibility of stacking as an emergent property. Strikingly, however, the combination of golgins conserved among diverse eukaryotes allows for the most detailed reconstruction of the organelle to date, showing a sophisticated Golgi with differentiated compartments and trafficking pathways in the common eukaryotic ancestor. -

Chromosomal Aberrations in Head and Neck Squamous Cell Carcinomas in Norwegian and Sudanese Populations by Array Comparative Genomic Hybridization
825-843 12/9/08 15:31 Page 825 ONCOLOGY REPORTS 20: 825-843, 2008 825 Chromosomal aberrations in head and neck squamous cell carcinomas in Norwegian and Sudanese populations by array comparative genomic hybridization ERIC ROMAN1,2, LEONARDO A. MEZA-ZEPEDA3, STINE H. KRESSE3, OLA MYKLEBOST3,4, ENDRE N. VASSTRAND2 and SALAH O. IBRAHIM1,2 1Department of Biomedicine, Faculty of Medicine and Dentistry, University of Bergen, Jonas Lies vei 91; 2Department of Oral Sciences - Periodontology, Faculty of Medicine and Dentistry, University of Bergen, Årstadveien 17, 5009 Bergen; 3Department of Tumor Biology, Institute for Cancer Research, Rikshospitalet-Radiumhospitalet Medical Center, Montebello, 0310 Oslo; 4Department of Molecular Biosciences, University of Oslo, Blindernveien 31, 0371 Oslo, Norway Received January 30, 2008; Accepted April 29, 2008 DOI: 10.3892/or_00000080 Abstract. We used microarray-based comparative genomic logical parameters showed little correlation, suggesting an hybridization to explore genome-wide profiles of chromosomal occurrence of gains/losses regardless of ethnic differences and aberrations in 26 samples of head and neck cancers compared clinicopathological status between the patients from the two to their pair-wise normal controls. The samples were obtained countries. Our findings indicate the existence of common from Sudanese (n=11) and Norwegian (n=15) patients. The gene-specific amplifications/deletions in these tumors, findings were correlated with clinicopathological variables. regardless of the source of the samples or attributed We identified the amplification of 41 common chromosomal carcinogenic risk factors. regions (harboring 149 candidate genes) and the deletion of 22 (28 candidate genes). Predominant chromosomal alterations Introduction that were observed included high-level amplification at 1q21 (harboring the S100A gene family) and 11q22 (including Head and neck squamous cell carcinoma (HNSCC), including several MMP family members). -

Knock-Out of ACBD3 Leads to Dispersed Golgi Structure, but Unaffected Mitochondrial Functions in HEK293 and Hela Cells
International Journal of Molecular Sciences Article Knock-Out of ACBD3 Leads to Dispersed Golgi Structure, but Unaffected Mitochondrial Functions in HEK293 and HeLa Cells Tereza Da ˇnhelovská 1 , Lucie Zdražilová 1 , Hana Štufková 1, Marie Vanišová 1, Nikol Volfová 1, Jana Kˇrížová 1 , OndˇrejKuda 2 , Jana Sládková 1 and Markéta Tesaˇrová 1,* 1 Department of Paediatrics and Inherited Metabolic Disorders, Charles University, First Faculty of Medicine and General University Hospital in Prague, 128 01 Prague, Czech Republic; [email protected] (T.D.); [email protected] (L.Z.); [email protected] (H.Š.); [email protected] (M.V.); [email protected] (N.V.); [email protected] (J.K.); [email protected] (J.S.) 2 Institute of Physiology, Academy of Sciences of the Czech Republic, 142 00 Prague, Czech Republic; [email protected] * Correspondence: [email protected] Abstract: The Acyl-CoA-binding domain-containing protein (ACBD3) plays multiple roles across the cell. Although generally associated with the Golgi apparatus, it operates also in mitochondria. In steroidogenic cells, ACBD3 is an important part of a multiprotein complex transporting cholesterol into mitochondria. Balance in mitochondrial cholesterol is essential for proper mitochondrial protein biosynthesis, among others. We generated ACBD3 knock-out (ACBD3-KO) HEK293 and HeLa cells and characterized the impact of protein absence on mitochondria, Golgi, and lipid profile. In ACBD3- Citation: Daˇnhelovská,T.; KO cells, cholesterol level and mitochondrial structure and functions are not altered, demonstrating Zdražilová, L.; Štufková, H.; that an alternative pathway of cholesterol transport into mitochondria exists. However, ACBD3- Vanišová, M.; Volfová, N.; Kˇrížová,J.; Kuda, O.; Sládková, J.; Tesaˇrová,M. -
Download Validation Data
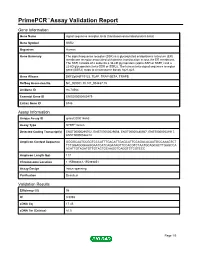
PrimePCR™Assay Validation Report Gene Information Gene Name signal sequence receptor, beta (translocon-associated protein beta) Gene Symbol SSR2 Organism Human Gene Summary The signal sequence receptor (SSR) is a glycosylated endoplasmic reticulum (ER) membrane receptor associated with protein translocation across the ER membrane. The SSR consists of 2 subunits a 34-kD glycoprotein (alpha-SSR or SSR1) and a 22-kD glycoprotein (beta-SSR or SSR2). The human beta-signal sequence receptor gene (SSR2) maps to chromosome bands 1q21-q23. Gene Aliases DKFZp686F19123, TLAP, TRAP-BETA, TRAPB RefSeq Accession No. NC_000001.10, NT_004487.19 UniGene ID Hs.74564 Ensembl Gene ID ENSG00000163479 Entrez Gene ID 6746 Assay Information Unique Assay ID qHsaCID0014663 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000295702, ENST00000529008, ENST00000480567, ENST00000531917, ENST00000526212 Amplicon Context Sequence GGGGCAATCCGGTCCCATTTGACATTGAGCATTCCAGACACAATGCCAAAGTCT TCTGGAGGGAAGGAATCATCAGATAGTTCCACGTCTAATGCAGCACTTGAGCCA ACATTGTAGATGTTGTACTGCAAGGTCAGGTCTCGTCCC Amplicon Length (bp) 117 Chromosome Location 1:155988061-155989851 Assay Design Intron-spanning Purification Desalted Validation Results Efficiency (%) 98 R2 0.9998 cDNA Cq 17.45 cDNA Tm (Celsius) 81.5 Page 1/5 PrimePCR™Assay Validation Report gDNA Cq Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report SSR2, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference -

Signal Peptide Peptidase‐Like 2C Impairs Vesicular Transport And
Article Signal peptide peptidase-like 2c impairs vesicular transport and cleaves SNARE proteins Alkmini A Papadopoulou1, Stephan A Müller2, Torben Mentrup3, Merav D Shmueli2,4,5, Johannes Niemeyer3, Martina Haug-Kröper1, Julia von Blume6, Artur Mayerhofer7, Regina Feederle2,8,9 , Bernd Schröder3,10 , Stefan F Lichtenthaler2,5,9 & Regina Fluhrer1,2,* Abstract Introduction Members of the GxGD-type intramembrane aspartyl proteases The high degree of compartmentalization in eukaryotic cells creates have emerged as key players not only in fundamental cellular a need for specific and precise protein trafficking. To meet this need, processes such as B-cell development or protein glycosylation, but cells have developed a complex system of vesicle transport that also in development of pathologies, such as Alzheimer’s disease or ensures safe sorting of cargo proteins, in particular between the dif- hepatitis virus infections. However, one member of this protease ferent compartments of the secretory pathway [1–3]. Vesicles origi- family, signal peptide peptidase-like 2c (SPPL2c), remains orphan nate from a donor membrane, translocate in a targeted manner, and and its capability of proteolysis as well as its physiological function get specifically tethered to the target membrane, before fusing with is still enigmatic. Here, we demonstrate that SPPL2c is catalytically it. Soluble N-ethylmaleimide-sensitive factor attachment protein active and identify a variety of SPPL2c candidate substrates using receptor (SNARE) proteins are known since three decades to medi- proteomics. The majority of the SPPL2c candidate substrates clus- ate specific membrane fusion [4,5]. So far, 38 SNARE proteins have ter to the biological process of vesicular trafficking. -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement. -

The Murine Orthologue of the Golgi-Localized TPTE Protein Provides Clues to the Evolutionary History of the Human TPTE Gene Family
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Hum Genet (2001) 109:569–575 DOI 10.1007/s004390100607 ORIGINAL INVESTIGATION Michel Guipponi · Caroline Tapparel · Olivier Jousson · Nathalie Scamuffa · Christophe Mas · Colette Rossier · Pierre Hutter · Paolo Meda · Robert Lyle · Alexandre Reymond · Stylianos E. Antonarakis The murine orthologue of the Golgi-localized TPTE protein provides clues to the evolutionary history of the human TPTE gene family Received: 5 June 2001 / Accepted: 17 August 2001 / Published online: 27 October 2001 © Springer-Verlag 2001 Abstract The human TPTE gene encodes a testis-spe- events. The Y chromosome copy of TPTE is a pseudo- cific protein that contains four potential transmembrane gene and is not therefore involved in the testis expression domains and a protein tyrosine phosphatase motif, and of this gene family. shows homology to the tumor suppressor PTEN/MMAC1. Chromosomal mapping revealed multiple copies of the TPTE gene present on the acrocentric chromosomes 13, Introduction 15, 21 and 22, and the Y chromosome. Zooblot analysis suggests that mice may possess only one copy of TPTE. We have recently identified a testis-specific cDNA, TPTE In the present study, we report the isolation and initial (Transmembrane Phosphatase with TEnsin homology), characterization of the full-length cDNA of the mouse ho- that encodes a predicted protein of 551 amino acids con- mologue Tpte. At least three different mRNA transcripts taining four potential transmembrane domains and a tyro- (Tpte.a, b, c) are produced via alternative splicing, encod- sine phosphatase motif (Chen H et al. -

Behavioral and Other Phenotypes in a Cytoplasmic Dynein Light Intermediate Chain 1 Mutant Mouse
The Journal of Neuroscience, April 6, 2011 • 31(14):5483–5494 • 5483 Cellular/Molecular Behavioral and Other Phenotypes in a Cytoplasmic Dynein Light Intermediate Chain 1 Mutant Mouse Gareth T. Banks,1* Matilda A. Haas,5* Samantha Line,6 Hazel L. Shepherd,6 Mona AlQatari,7 Sammy Stewart,7 Ida Rishal,8 Amelia Philpott,9 Bernadett Kalmar,2 Anna Kuta,1 Michael Groves,3 Nicholas Parkinson,1 Abraham Acevedo-Arozena,10 Sebastian Brandner,3,4 David Bannerman,6 Linda Greensmith,2,4 Majid Hafezparast,9 Martin Koltzenburg,2,4,7 Robert Deacon,6 Mike Fainzilber,8 and Elizabeth M. C. Fisher1,4 1Department of Neurodegenerative Disease, 2Sobell Department of Motor Science and Movement Disorders, 3Division of Neuropathology, and 4Medical Research Council (MRC) Centre for Neuromuscular Diseases, University College London (UCL) Institute of Neurology, London WC1N 3BG, United Kingdom, 5MRC National Institute for Medical Research, London NW7 1AA, United Kingdom, 6Department of Experimental Psychology, University of Oxford, Oxford OX1 3UD, United Kingdom, 7UCL Institute of Child Health, London WC1N 1EH, United Kingdom, 8Department of Biological Chemistry, Weizmann Institute of Science, 76100 Rehovot, Israel, 9School of Life Sciences, University of Sussex, Brighton BN1 9QG, United Kingdom, and 10MRC Mammalian Genetics Unit, Harwell OX11 ORD, United Kingdom The cytoplasmic dynein complex is fundamentally important to all eukaryotic cells for transporting a variety of essential cargoes along microtubules within the cell. This complex also plays more specialized roles in neurons. The complex consists of 11 types of protein that interact with each other and with external adaptors, regulators and cargoes. Despite the importance of the cytoplasmic dynein complex, weknowcomparativelylittleoftherolesofeachcomponentprotein,andinmammalsfewmutantsexistthatallowustoexploretheeffects of defects in dynein-controlled processes in the context of the whole organism. -

Multifaceted Polo-Like Kinases: Drug Targets and Antitargets for Cancer Therapy
REVIEWS Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy Klaus Strebhardt Abstract | The polo-like kinase 1 (PLK1) acts in concert with cyclin-dependent kinase 1–cyclin B1 and Aurora kinases to orchestrate a wide range of critical cell cycle events. Because PLK1 has been preclinically validated as a cancer target, small-molecule inhibitors of PLK1 have become attractive candidates for anticancer drug development. Although the roles of the closely related PLK2, PLK3 and PLK4 in cancer are less well understood, there is evidence showing that PLK2 and PLK3 act as tumour suppressors through their functions in the p53 signalling network, which guards the cell against various stress signals. In this article, recent insights into the biology of PLKs will be reviewed, with an emphasis on their role in malignant transformation, and progress in the development of small-molecule PLK1 inhibitors will be examined. More than two decades ago polo, the founding member Early observations on the overexpression of PLK1 of the family of polo-like kinases (PLKs), was identified in human tumours12 and on the inhibition of cellu- as having an essential role in the ordered execution of lar proliferation by microinjecting antibodies against mitotic events in Drosophila melanogaster 1,2. Since the PLK1 into HeLa cells13 initiated a series of follow-on discovery of polo, a wealth of functional information on studies using a broad spectrum of inhibitors (for exam- PLKs has been collected in a wide phylogenetic space. ple, dominant-negative forms of PLK1, antisense oli- Five mammalian PLK family members have been iden- gonucleotides and small interfering RNAs) aiming to tified so far, PLK1 (also known as STPK13), PLK2 (also evaluate PLK1 as a potential target for the treatment known as SNK), PLK3 (also known as CNK, FNK and of cancer14–17. -

Datasheet: MCA3923Z Product Details
Datasheet: MCA3923Z Description: MOUSE ANTI HUMAN ACBD3:Preservative Free Specificity: ACBD3 Format: Preservative Free Product Type: Monoclonal Antibody Clone: 2G2 Isotype: IgG1 Quantity: 0.1 mg Product Details Applications This product has been reported to work in the following applications. This information is derived from testing within our laboratories, peer-reviewed publications or personal communications from the originators. Please refer to references indicated for further information. For general protocol recommendations, please visit www.bio-rad-antibodies.com/protocols. Yes No Not Determined Suggested Dilution Immunohistology - Paraffin (1) 0.1 - 10 ug/ml Western Blotting Immunofluorescence 0.1 - 10 ug/ml Where this product has not been tested for use in a particular technique this does not necessarily exclude its use in such procedures. Suggested working dilutions are given as a guide only. It is recommended that the user titrates the product for use in their own system using appropriate negative/positive controls. (1)This product requires antigen retrieval using heat treatment prior to staining of paraffin sections.Sodium citrate buffer pH 6.0 is recommended for this purpose. Target Species Human Species Cross Reacts with: Rat, Mouse Reactivity N.B. Antibody reactivity and working conditions may vary between species. Product Form Purified IgG - liquid Preparation Purified IgG prepared by affinity chromatography on Protein A Buffer Solution Phosphate buffered saline Preservative None present Stabilisers Approx. Protein Ig concentration 0.5 mg/ml Concentrations Immunogen Recombinant protein corresponding to aa 73-172 of human ACBD3 Page 1 of 3 External Database Links UniProt: Q9H3P7 Related reagents Entrez Gene: 64746 ACBD3 Related reagents Synonyms GCP60, GOCAP1, GOLPH1 Fusion Partners Spleen cells from immunised Balb/c mice were fused with cells from the Sp2/0 myeloma cell line. -

Tyr198 Is the Essential Autophosphorylation Site for STK16 Localization and Kinase Activity
International Journal of Molecular Sciences Article Tyr198 is the Essential Autophosphorylation Site for STK16 Localization and Kinase Activity 1,2, 3, 1 1,2,4, Junjun Wang y, Juanjuan Liu y, Xinmiao Ji and Xin Zhang * 1 High Magnetic Field Laboratory, Key Laboratory of High Magnetic Field and Ion Beam Physical Biology, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, China; [email protected] (J.W.); xinmiaoji@hmfl.ac.cn (X.J.) 2 Science Island Branch of Graduate School, University of Science and Technology of China, Hefei 230026, China 3 School of Life Sciences, Anhui University, Hefei 230601, China; [email protected] 4 Institutes of Physical Science and Information Technology, Anhui University, Hefei 230601, China * Correspondence: xinzhang@hmfl.ac.cn The authors contributed equally to this work. y Received: 27 August 2019; Accepted: 25 September 2019; Published: 30 September 2019 Abstract: STK16, reported as a Golgi localized serine/threonine kinase, has been shown to participate in multiple cellular processes, including the TGF-β signaling pathway, TGN protein secretion and sorting, as well as cell cycle and Golgi assembly regulation. However, the mechanisms of the regulation of its kinase activity remain underexplored. It was known that STK16 is autophosphorylated at Thr185, Ser197, and Tyr198 of the activation segment in its kinase domain. We found that STK16 localizes to the cell membrane and the Golgi throughout the cell cycle, but mutations in the auto-phosphorylation sites not only alter its subcellular localization but also affect its kinase activity. In particular, the Tyr198 mutation alone significantly reduced the kinase activity of STK16, abolished its Golgi and membrane localization, and affected the cell cycle progression.