The Crystal Chemistry of Julgoldite-Fe3+ from Bombay, India, Studied Using Synchrotron X-Ray Powder Diffraction and 57Fe Mössbauer Spectroscopy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pumpellyite-(Al), a New Mineral from Bertrix, Belgian Ardennes
Eur. J. Mineral. 2007, 19, 247–253 Pumpellyite-(Al), a new mineral from Bertrix, Belgian Ardennes FRED´ ERIC´ HATERT1,*,MARCO PASERO 2,NATALE PERCHIAZZI2 and THOMAS THEYE3 1 LaboratoiredeMin´eralogie, D´epartement de G´eologie, Bˆatiment B18, Universit´edeLi`ege, 4000 Li`ege, Belgium * Corresponding author, e-mail: [email protected] 2 Dipartimento di Scienze della Terra, Universit`a degli Studi di Pisa, Via S. Maria 53, 56126 Pisa, Italy 3 Institut für Mineralogie und Kristallchemie, Universität Stuttgart, Azenbergstraße 18, 70174 Stuttgart, Germany 2+ Abstract: Pumpellyite-(Al), ideally Ca2(Al,Fe ,Mg)Al2(SiO4)(Si2O7)(OH,O)2·H2O, is a newly approved mineral species from Bertrix, Ardennes mountains, Belgium. It occurs as radiating fibrous aggregates reaching 5 mm in diameter, constituted by acicular crystals associated with calcite, K-feldspar and chlorite. Pumpellyite-(Al) is transparent to translucent and exhibits an emerald-green to white colour, sometimes with bluish tinges. The lustre is vitreous and the streak is colourless. The mineral is non-fluorescent, brittle, and shows a perfect {100} cleavage. The estimated Mohs hardness is 5½, and the calculated density is 3.24 g/cm3. Pumpellyite-(Al) is biaxial positive, [ = 1.678(2), q = 1.680(2), * = 1.691(1) ( † = 590 nm), colourless in thin section, 2V = 46°, Y = b, no dispersion. Electron-microprobe analyses gave SiO2 37.52, Al2O3 25.63, MgO 1.99, FeO 4.97, MnO 0.11, CaO 23.21, BaO 0.01, Na2O 0.03, K2O 0.02, H2Ocalc. 6.71, total 100.20 wt. %. The resulting empirical formula, calculated on the basis of 2+ 8 cations, is (Ca1.99Na0.01) 7 2.00(Al0.42Fe 0.33Mg0.24Mn0.01) 7 1.00Al2.00(SiO4)(Si2O7)(OH)2.42 · 0.58H2O. -

By John Jeffrey Josephson
MINERAL CHEMISTRY AND PHASE RELATIONS IN VOLCANOGENIC SEDIMENTS METAMORPHOSED TO PUMPELLYITE-ACTINOLITE FACIES, CHATHAM ISLAND, NEW ZEALAND PLATEAU, SOUTHWEST PACIFIC BY JOHN JEFFREY JOSEPHSON CONTRIBUTION NO. 55 DEPARTMENT OF GEOLOGY a GEOGRAPHY UNIVERSITY OF MASSACHUSETTS AMHERST, MASSACHUSETTS THE MINERAL CHEMISTRY AND PHASE RELATIONS IN VOLCANOGENIC SEDIMENTS METAMORPHOSED TO THE PUMPELLYITE-ACTINOLITE FACIES, CHATHAM ISLAND, NEW ZEALAND PLATEAU, SOUTHWEST PACIFIC (M.S. Thesis) by John Jeffrey Josephson Contribution No. 55 Department of Geology and Geography University of Massachusetts Amherst, Massachusetts June, 1985 iii TABLE OF CONTENTS Page ABSTRACT •.... 1 INTRODUCTION. 3 Regional Setting .. 3 Rock Types and Stratigraphy .. 5 Structural and Metamorphic History. 8 Purpose of Study ......•... 9 Acknowledgments. 9 PETROGRAPHY ...•.••. 9 Introduction. 9 Quartz-Albite Schists .. 15 Black Argillaceous Schist .. 1 7 Greenschists •.......... 1 7 Garnet-bearing Schist .. 20 Relations Between Tectonic Fabrics and Metamorphic Recrystallization .. 20 WHOLE ROCK GEOCHEMISTRY •. 22 Analytical Methods .. 22 Introduction ...... 22 Major Element Data .. 23 Trace Element Data. 23 Discussion. 26 MINERALOGY ..•.... 30 Analytical Procedure. 30 White Mica ......•..... 30 Thin section description ... 30 Chemistry .. 33 Chlorite ....... 40 Thin section description. 40 Chemistry ...•... 40 Epidote-Clinozoisite. 50 Thin section description •. 50 Chemistry .. 50 Pumpellyite ..... 54 Thin section description ... • •••••••••• 54 Chemistry •...............•... -

Alphabetical List
LIST L - MINERALS - ALPHABETICAL LIST Specific mineral Group name Specific mineral Group name acanthite sulfides asbolite oxides accessory minerals astrophyllite chain silicates actinolite clinoamphibole atacamite chlorides adamite arsenates augite clinopyroxene adularia alkali feldspar austinite arsenates aegirine clinopyroxene autunite phosphates aegirine-augite clinopyroxene awaruite alloys aenigmatite aenigmatite group axinite group sorosilicates aeschynite niobates azurite carbonates agate silica minerals babingtonite rhodonite group aikinite sulfides baddeleyite oxides akaganeite oxides barbosalite phosphates akermanite melilite group barite sulfates alabandite sulfides barium feldspar feldspar group alabaster barium silicates silicates albite plagioclase barylite sorosilicates alexandrite oxides bassanite sulfates allanite epidote group bastnaesite carbonates and fluorides alloclasite sulfides bavenite chain silicates allophane clay minerals bayerite oxides almandine garnet group beidellite clay minerals alpha quartz silica minerals beraunite phosphates alstonite carbonates berndtite sulfides altaite tellurides berryite sulfosalts alum sulfates berthierine serpentine group aluminum hydroxides oxides bertrandite sorosilicates aluminum oxides oxides beryl ring silicates alumohydrocalcite carbonates betafite niobates and tantalates alunite sulfates betekhtinite sulfides amazonite alkali feldspar beudantite arsenates and sulfates amber organic minerals bideauxite chlorides and fluorides amblygonite phosphates biotite mica group amethyst -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -
![Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2crcr2[Sio4][Si2o6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite](https://docslib.b-cdn.net/cover/6642/chromium-members-of-the-pumpellyite-group-shuiskite-cr-ca2crcr2-sio4-si2o6-oh-oh-2o-a-new-mineral-and-shuiskite-mg-a-new-species-name-for-shuiskite-2006642.webp)
Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2crcr2[Sio4][Si2o6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite
minerals Article Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite Inna Lykova 1,*, Dmitry Varlamov 2 , Nikita Chukanov 3, Igor Pekov 4, Dmitry Belakovskiy 5 , 6, 4 7 Oleg Ivanov y, Natalia Zubkova and Sergey Britvin 1 Canadian Museum of Nature, P.O. Box 3443, Station D, Ottawa, ON K1P 6P4, Canada 2 Institute of Experimental Mineralogy, Russian Academy of Sciences, Chernogolovka, Moscow Oblast 142432, Russia; [email protected] 3 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, Moscow Oblast 142432, Russia; [email protected] 4 Faculty of Geology, Moscow State University, Vorobyovy Gory, Moscow 119991, Russia; [email protected] (I.P.); [email protected] (N.Z.) 5 Fersman Mineralogical Museum, Russian Academy of Sciences, Leninsky Prospekt 18-2, Moscow 119071, Russia; [email protected] 6 Zavaritsky Institute of Mineralogy and Geochemistry, Ural Branch of the Russian Academy of Sciences, Ekaterinburg 620016, Russia; [email protected] 7 Department of Crystallography, Institute of Earth Sciences, St. Petersburg State University, University Emb. 7/9, St. Petersburg 199034, Russia; [email protected] * Correspondence: [email protected] Deceased 01 February 2020. y Received: 7 April 2020; Accepted: 23 April 2020; Published: 26 April 2020 Abstract: A new pumpellyite-group mineral shuiskite-(Cr), ideally Ca2CrCr2[SiO4][Si2O6(OH)] (OH)2O, was found at the Rudnaya mine, Glavnoe Saranovskoe deposit, Middle Urals, Russia. It occurs on the walls of 0.5 to 1 cm thick fractures in chromitite, filled with calcite, Cr-bearing clinochlore, and uvarovite. -

The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland
minerals Article The Behaviour of Siderite Rocks in an Experimental Imitation of Pyrometamorphic Processes in Coal-Waste Fires: Upper and Lower Silesian Case, Poland Łukasz Kruszewski 1 and Justyna Ciesielczuk 2,* 1 Institute of Geological Sciences, Polish Academy of Sciences (ING PAN), Twarda 51/55, 00-818 Warszawa, Poland; [email protected] 2 Faculty of Natural Sciences, University of Silesia, B˛edzi´nska60, 41-205 Sosnowiec, Poland * Correspondence: [email protected] Received: 18 May 2020; Accepted: 26 June 2020; Published: 29 June 2020 Abstract: Little is known of the influence of fluxes on the nature and the intensity of burning in coal-waste heaps. To gain some insight, two siderite samples, one each from coal-mining waste heaps in Upper- and Lower Silesian Coal Basins (Poland), were heated under identical conditions in a thermal chamber coupled to a powder X-ray diffractometer. Differences in the behaviour of siderite phase and the products of its decomposition, mainly magnetite, wüstite, and olivine, are discussed. The waste heaps sampled underwent self-heating and self-ignition catalysed by fluxes. Though the samples are unlikely to be truly representative of the Silesian basins, the heterogeneous behaviour they displayed on heating merits description and explanation, as siderite is an important widely known flux in pyrometamorphic processes. Keywords: thermal chamber experiment; Powder X-ray Diffraction; pyrometamorphism; siderite; wüstite; magnetite; graphite 1. Introduction In coal basins in Poland, siderite occurs mainly as concretions of different sizes and shapes within mudstones, coaly shales, sandstones, and conglomerates hosting coal seams. As is common worldwide, these seams can undergo spontaneous combustion influenced by numerous external and internal factors [1–6]. -
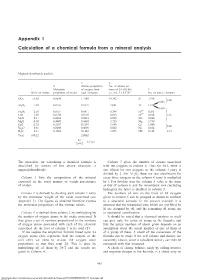
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

And Merguerian, Charles, 2015, Western Connecticut's
MORITZ AND MERGUERIAN C1-1 WESTERN CONNECTICUT’S VARIED MINERAL-FORMING GEOENVIRONMENTS Harold Moritz, 15 Geoffrey Road, East Haddam, CT 06423-1015 [email protected] Charles Merguerian, Duke Geological Laboratory, 55 Spongia Road, Stone Ridge, NY 12484 [email protected] INTRODUCTION Connecticut boasts a broad range of mineral forming geoenvironments and thus a long list of mineral species that have long attracted miners, researchers and collectors. The mindat.org Connecticut page lists 770 mineral species, series, group or variety names. The state is so rich in minerals, both in number and distribution, that any exposure will reveal something of interest and often quite different from others nearby. This great variety and distribution was noticed right away by colonists, who in their drive to find riches scrutinized every outcrop in the increasingly denuded landscape using what was essentially a haphazard, bottom up approach to resource prospecting. They were missing “the big picture” that would allow a predictive, top down approach, therefore, much of their effort was wasted due to lack of understanding. Today we can make sense of the variety of mineral occurrences by viewing them in the context of the geoenvironments they occur in, which resulted from the state’s long and complex geologic history. That history, of repeated terrane accretion with coeval compressive deformation, metamorphism and plutonism from the Proterozoic through Permian, to extension and coeval sedimentation, volcanism and hydrothermal activity in the early Mesozoic, is well told elsewhere and is alluded to here only as necessary. Given the regional scale of these events and tectonic terranes, most major types of mineral forming geoenvironments in the state are not unique to one locality, thus a selection from each can represent a range of places, but with local, sometimes unique variations. -

List of Mineral Symbols
THE CANADIAN MINERALOGIST LIST OF SYMBOLS FOR ROCK- AND ORE-FORMING MINERALS (January 1, 2021) ____________________________________________________________________________________________________________ Ac acanthite Ado andorite Asp aspidolite Btr berthierite Act actinolite Adr andradite Ast astrophyllite Brl beryl Ae aegirine Ang angelaite At atokite Bll beryllonite AeAu aegirine-augite Agl anglesite Au gold Brz berzelianite Aen aenigmatite Anh anhydrite Aul augelite Bet betafite Aes aeschynite-(Y) Ani anilite Aug augite Bkh betekhtinite Aik aikinite Ank ankerite Aur auricupride Bdt beudantite Akg akaganeite Ann annite Aus aurostibite Beu beusite Ak åkermanite An anorthite Aut autunite Bch bicchulite Ala alabandite Anr anorthoclase Aw awaruite Bt biotite* Ab albite Atg antigorite Axn axinite-(Mn) Bsm bismite Alg algodonite Sb antimony Azu azurite Bi bismuth All allactite Ath anthophyllite Bdl baddeleyite Bmt bismuthinite Aln allanite Ap apatite* Bns banalsite Bod bohdanowiczite Alo alloclasite Arg aragonite Bbs barbosalite Bhm böhmite Ald alluaudite Ara aramayoite Brr barrerite Bor boralsilite Alm almandine Arf arfvedsonite Brs barroisite Bn bornite Alr almarudite Ard argentodufrénoysite Blt barylite Bou boulangerite Als alstonite Apn argentopentlandite Bsl barysilite Bnn bournonite Alt altaite Arp argentopyrite Brt baryte, barite Bow bowieite Aln alunite Agt argutite Bcl barytocalcite Brg braggite Alu alunogen Agy argyrodite Bss bassanite Brn brannerite Amb amblygonite Arm armangite Bsn bastnäsite Bra brannockite Ams amesite As arsenic -

NEW MINERAL NAMES Mrcnen'r Frprscnsn
THE AMERICAN MINERALOGIST, VOL. 56, NOVEMBER-DECEMBER, 1971 NEW MINERAL NAMES Mrcnen'r Frprscnsn Ewaldite (also new data on mackelveyite) G,rsnrnllr Dowmev aNo J. D. H. Domrav (1971) Ewaldite, a new barium calcium car- bonate. r. occurrence of ewaldite in syntactic intergrowth with mackelveyite. (with analysis by M. H. Hey) Tschermaks Mineral. petrogr. Mitt. lS, 1g5-200. G,q.ettnr,r.n DoNNav amo H. PrasroN (1971) Ewaldite, a new barium calcium carbonate. If. Its crystal structure. Tschermaks Minad. petrogr. MitL lS,2Ol-2I2. ewaldite, is the major constituent of the polycrystals studied by Desautels (Amer. Mineral. 52, 860-8&, [1967] under the name mackelveyite. rt is bluish green and crystallizes in P6smc with a 5.284+0.ffi7, and c 12.78+0.01 A. Microprobe examination of four individuals consisting of about equal parts of ewaldite and mackelveyite showed the elements Ba, ca, y, and Na to be uniformly distributed, indicating that ewaldite and mackelveyite are a dimorphous pair, ,,except for possibly different water content." (The water content reported for mackelveyite, 4.g or s.0/6lna<l been determined "by difierence.") The results of microchemical analysis of selected ewaldite crystals, supplemented by determination of the structure, lead to the formula Bar.oo(Cao.reRZo.zeNas.15Ks.11Sro.o:UoozIoor)(COr)r,with Z:2 and density (calc.) 3.37 g/cm3 (meas.) 3.25+0.05 g/cm3. The optical properties of ewaldite, determined on a polycrystal showing only a minimal amount of mackelveyilg, are: o(Na) : 1.646 + 0.003, e11.572, maximum birefringence thus at least 0.074, pleochroic, O dark bluish green, E pale yellowish green. -

1 Michael Fleischer and Mary Woodruff Open-File Report 88-689
U.S. DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY THE FORD-FLEISCHER FILE OF MINERALOGICAL REFERENCES, 1982-1987 INCLUSIVE by Michael Fleischer 1 and Mary Woodruff Open-File Report 88-689 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards. Dept. Mineral Sciences, National Museum of Natural History, Smithsonian Institution, Washington, D.C. 20560 2 U.S. Geological Survey, MS 959, 12201 Sunrise Valley, Reston, VA 22092 1988 The Ford-Fleischer File of Mineralogical References 1982-1987 Inclusive In 1916, Prof. W.E. Ford of Yale University, having just published the Third Appendix to Dana's System of Mineralogy, 6th Edition, began to plan for the 7th Edition. He decided to create a file with a separate folder for each mineral (or for each mineral group) into which he would place a citation to any paper that contained data that should be considered in the revision of the 6th Edition. The file was maintained in duplicate with one copy going to Harvard University. In the early 1930's Palache, Berman, and Frondel at Harvard were designated chief revisionists. Assistants for the project included C.W. Wolfe and M.A. Peacock who were to gather crystallographic data at Harvard; and their counterpart, Michael Fleischer, who was to collect and evaluate chemical data at Yale. After Prof. Ford's death in March 1939, one set of his files came to the U.S. Geological Survey, and the literature has been covered since then by Michael Fleischer. Copies of the literature survey are maintained at the U.S. -

AND ORE-FORMING MINERALS (December 30, 2019) ______
THE CANADIAN MINERALOGIST LIST OF SYMBOLS FOR ROCK- AND ORE-FORMING MINERALS (December 30, 2019) ____________________________________________________________________________________________________________ Ac acanthite Adr andradite Ast astrophyllite Brl beryl Act actinolite Ang angelaite At atokite Bll beryllonite Ae aegirine Agl anglesite Au gold Brz berzelianite Aen aenigmatite Anh anhydrite Aul augelite Bet betafite Aes aeschynite-(Y) Ani anilite Aug augite Bkh betekhtinite Aik aikinite Ank ankerite Aur auricupride Bdt beudantite Akg akaganeite Ann annite Aus aurostibite Beu beusite Ak åkermanite An anorthite Aut autunite Bch bicchulite Ala alabandite Anr anorthoclase Aw awaruite Bt biotite* Ab albite Atg antigorite Axn axinite-(Mn) Bsm bismite Alg algodonite Sb antimony Azu azurite Bi bismuth All allactite Ath anthophyllite Bdl baddeleyite Bmt bismuthinite Aln allanite Ap apatite* Bns banalsite Bod bohdanowiczite Alo alloclasite Arg aragonite Bbs barbosalite Bhm böhmite Ald alluaudite Ara aramayoite Brr barrerite Bor boralsilite Alm almandine Arf arfvedsonite Brs barroisite Bn bornite Alr almarudite Ard argentodufrénoysite Blt barylite Bou boulangerite Als alstonite Apn argentopentlandite Bsl barysilite Bnn bournonite Alt altaite Arp argentopyrite Brt baryte, barite Bow bowieite Aln alunite Agt argutite Bcl barytocalcite Brg braggite Alu alunogen Agy argyrodite Bss bassanite Brn brannerite Amb amblygonite Arm armangite Bsn bastnäsite Bra brannockite Ams amesite As arsenic Bau baumstarkite Bnt braunite Amp amphibole* Ass arseniosiderite