Ongoing Outbreak of COVID-19 in Iran: Challenges and Signs of Concern with Under-Reporting of Prevalence and Deaths
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Civil Aviation Organization Aviation Data and Analysis Seminar (Tehran, Iran I.R, 20
International Civil Aviation Organization Aviation Data and Analysis Seminar (Tehran, Iran I.R, 20 - 23 February 2017) LIST OF PARTICIPANTS 20 February 2017 NAME TITLE & ADDRESS STATES ALGERIA Ms. Kahina Loucif Commercial Director Airport Services Management Establishment ALGERIA Ms. Lamia Ouali Technical Framework Airport Services Management Establishment ALGERIA INDIA Mr. Praveen Kumar Srivastava Deputy Director (ISS) Directorate general of Civil Aviation (DGCA) New Delhi, INDIA ISLAMIC REPUBLIC OF IRAN Mr. Mortaza Dehghan Vice President of Aeronautical and International Affairs Civil Aviation Organization Tehran - ISLAMIC REPUBLIC OF IRAN Mr. Arash Khodai Vice President Civil Aviation Organization Tehran - ISLAMIC REPUBLIC OF IRAN Mr. Vahid Ezoji Vice President of Administrative Department Civil Aviation Organization Tehran - ISLAMIC REPUBLIC OF IRAN Mr. Hassanali Shahbazilar Representative of I.R. of Iran to the ICAO ICAO HQ Montreal - CANADA Mr. Morad Esmaili Director General for Legal and International Affairs Tehran - ISLAMIC REPUBLIC OF IRAN Mr. Mohammad Saeed Sharafi General Director for Aerodrome and ANS oversight Civil Aviation Organization Tehran - ISALAMIC REPUBLIC OF IRAN - 2 - NAME TITLE & ADDRESS Rahmatoolah Firoozipour Director General Civil Aviation Organization Tehran - ISALAMIC REPUBLIC OF IRAN Mr. Meisam Shaker Arani Assistance Director for Aerodrome and ANS Oversight Civil Aviation Organization Tehran - ISLAMIC REPUBLIC OF IRAN Mrs. Fahimeh Sarhadi Deputy Director for International Affairs Civil Aviation Organization Tehran - ISALAMIC REPUBLIC OF IRAN Mr. Mehdi Zand Senior Expert for International Affairs Civil Aviation Organization Tehran - ISLAMIC REPUBLIC OF IRAN Mr. Ramin Amintinat Senior Expert for International Affairs Assurance Manager Tehran - ISLAMIC REPUBLIC OF IRAN Ms. Yasaman Allameh Senior Expert for International Affairs Civil Aviation Organization Tehran - ISLAMIC REPUBLIC OF IRAN Mr. -

Vea Un Ejemplo
3 To search aircraft in the registration index, go to page 178 Operator Page Operator Page Operator Page Operator Page 10 Tanker Air Carrier 8 Air Georgian 20 Amapola Flyg 32 Belavia 45 21 Air 8 Air Ghana 20 Amaszonas 32 Bering Air 45 2Excel Aviation 8 Air Greenland 20 Amaszonas Uruguay 32 Berjaya Air 45 748 Air Services 8 Air Guilin 20 AMC 32 Berkut Air 45 9 Air 8 Air Hamburg 21 Amelia 33 Berry Aviation 45 Abu Dhabi Aviation 8 Air Hong Kong 21 American Airlines 33 Bestfly 45 ABX Air 8 Air Horizont 21 American Jet 35 BH Air - Balkan Holidays 46 ACE Belgium Freighters 8 Air Iceland Connect 21 Ameriflight 35 Bhutan Airlines 46 Acropolis Aviation 8 Air India 21 Amerijet International 35 Bid Air Cargo 46 ACT Airlines 8 Air India Express 21 AMS Airlines 35 Biman Bangladesh 46 ADI Aerodynamics 9 Air India Regional 22 ANA Wings 35 Binter Canarias 46 Aegean Airlines 9 Air Inuit 22 AnadoluJet 36 Blue Air 46 Aer Lingus 9 Air KBZ 22 Anda Air 36 Blue Bird Airways 46 AerCaribe 9 Air Kenya 22 Andes Lineas Aereas 36 Blue Bird Aviation 46 Aereo Calafia 9 Air Kiribati 22 Angkasa Pura Logistics 36 Blue Dart Aviation 46 Aero Caribbean 9 Air Leap 22 Animawings 36 Blue Islands 47 Aero Flite 9 Air Libya 22 Apex Air 36 Blue Panorama Airlines 47 Aero K 9 Air Macau 22 Arab Wings 36 Blue Ridge Aero Services 47 Aero Mongolia 10 Air Madagascar 22 ARAMCO 36 Bluebird Nordic 47 Aero Transporte 10 Air Malta 23 Ariana Afghan Airlines 36 Boliviana de Aviacion 47 AeroContractors 10 Air Mandalay 23 Arik Air 36 BRA Braathens Regional 47 Aeroflot 10 Air Marshall Islands 23 -

Global Volatility Steadies the Climb
WORLD AIRLINER CENSUS Global volatility steadies the climb Cirium Fleet Forecast’s latest outlook sees heady growth settling down to trend levels, with economic slowdown, rising oil prices and production rate challenges as factors Narrowbodies including A321neo will dominate deliveries over 2019-2038 Airbus DAN THISDELL & CHRIS SEYMOUR LONDON commercial jets and turboprops across most spiking above $100/barrel in mid-2014, the sectors has come down from a run of heady Brent Crude benchmark declined rapidly to a nybody who has been watching growth years, slowdown in this context should January 2016 low in the mid-$30s; the subse- the news for the past year cannot be read as a return to longer-term averages. In quent upturn peaked in the $80s a year ago. have missed some recurring head- other words, in commercial aviation, slow- Following a long dip during the second half Alines. In no particular order: US- down is still a long way from downturn. of 2018, oil has this year recovered to the China trade war, potential US-Iran hot war, And, Cirium observes, “a slowdown in high-$60s prevailing in July. US-Mexico trade tension, US-Europe trade growth rates should not be a surprise”. Eco- tension, interest rates rising, Chinese growth nomic indicators are showing “consistent de- RECESSION WORRIES stumbling, Europe facing populist backlash, cline” in all major regions, and the World What comes next is anybody’s guess, but it is longest economic recovery in history, US- Trade Organization’s global trade outlook is at worth noting that the sharp drop in prices that Canada commerce friction, bond and equity its weakest since 2010. -

Standard Tour
Standard Tour 8 days 7 nights from 12 November to 19 November DAY 1 12 November Saturday Meet up in Tehran IKA airport on arrivals Transfer to hotel Hotel: Mehrabad Airport Hotel or similar (3 star) DAY 2 13 November Sunday Flying the An-74 from Tehran to Destination TBD Afternoon free day in Tehran Hotel: Mehrabad Airport Hotel or similar (3 star) DAY 3 14 November Monday Fly Iran Air or Qeshm Air to Isfahan in the morning. Morning tea at the historic Hotel Abassi Full day sightseeing at Isfahan, ancient Persian capital. See the best of Persia and enjoy the ancient culture. We will fly back to Tehran in the late evening. Hotel: Mehrabad Airport Hotel or similar (3 star) DAY 4 15 November Tuesday Fly the last B727-200 pax model to Mashhad and back. Lunch with Iran Air crew and possibility (yet to be confirmed) to have access visit to Iran Air facility. Hotel: Mehrabad Airport Hotel or similar (3 star) DAY 5 16 November Wednesday Flying to Shiraz Sightseeing in "Pink Mosque" and Persepolis Return to Tehran in the evening Hotel: Mehrabad Airport Hotel or similar (3 star) DAY 6 17 November Thursday Flying to Kish Island in the south Iran for the bi-annual Iran Air Show Full day spent in the air show with full photography access. Hotel: On Kish Island TBA DAY 7 18 November Friday Morning spent in Iran Air Show on Kish Island Flying in the afternoon to Tehran In the evening, transfer to IKA airport to your home country. -

Hej, UD Avråder Sedan Den 2 Mars Från Samtliga Resor Till Iran. Avrådansbeslutet Fattades På Basis Av Omfattande Spridning A
Hej, UD avråder sedan den 2 mars från samtliga resor till Iran. Avrådansbeslutet fattades på basis av omfattande spridning av coronaviruset Covid-19 i Iran. Angränsande länder har stängt sina gränser till Iran och ett antal flygbolag har tillfälligt upphört med att flyga till och från Iran. Transportstyrelsen har sedan den 2 mars tillfälligt dragit in det svenska trafiktillståndet för Iran Air. Vi förstår att det finns en stor oro just nu kring möjligheterna att resa hem till Sverige och att beslutet att återkalla trafiktillståndet för Iran Air till Sverige har gjort det svårare att lämna Iran. Det finns fortfarande möjlighet att flyga med vanliga kommersiella flighter. Vi rekommenderar svenskar som vill resa från Iran att kontakta resebyråer och flygbolag. Utöver de reguljära flighterna finns möjlighet att flyga med ett extrainsatt plan med Mahan Air till Barcelona den 12 mars. Flighten kommer endast att genomföras om tillräckligt många har anmält sig. Ambassaden kan inte garantera att flighten genomförs eller ta ansvar för eventuella ändringar och kan inte heller garantera att de som anmäler sitt intresse faktiskt får plats på flyget. Biljettpris sätts av Mahan Air. Betalning är ett personligt ansvar. Vänligen kontakta ambassaden senast måndag den 9 mars kl. 12.00 om ni är intresserade av att anmäla er till flighten till Barcelona. Meddela födelsedatum, passnummer och gender. OBSERVERA! Anmälan kan endast göras till: [email protected] . För att erbjuda en överblick över de restriktioner och möjligheter som föreligger följer nedan information sammanställd av WHO i Iran. Se särskilt de gulmarkerade flygbolagen, som i skrivande stund uppges ha bokningsbara platser under kommande veckor. -

Trend of COVID‐19 I.R. IRAN Update 466 / 31 May 2021
No. 466 / 31 May 2021 New Lab Total Lab Confirmed Cases New Deaths Confirmed Cases Total Recoveries Total Deaths IRAN 11,042↑(+2,166) 217↑(+19) 2,913,136 2,458,684 80,156 Trend of COVID‐19 I.R. IRAN Update Figure 1 - Trend of COVID-19 Laboratory-Confirmed Cases and Deaths, 19 February 2020 - 31 May 2021 600 500 400 300 No. of Cases No. of Deaths 200 100 0 19-Feb-20 19-Mar-20 19-Apr-20 19-May-20 19-Jun-20 19-Jul-20 19-Aug-20 19-Sep-20 19-Oct-20 19-Nov-20 19-Dec-20 19-Jan-21 19-Feb-21 19-Mar-21 19-Apr-21 19-May-21 Laboratory Confirmed Cases Deaths Number of cases admitted to ICU: 4,160 ↓ (-59) Figure 2 - COVID-19 Patients in Intensive Care, 26 March 2020 - 31 May 2021 6,000 5,500 5,000 4,500 4,000 3,500 3,000 2,500 2,000 0 26-Mar-20 26-Apr-20 26-May-20 26-Jun-20 26-Jul-20 26-Aug-20 26-Sep-20 26-Oct-20 26-Nov-20 26-Dec-20 26-Jan-21 26-Feb-21 26-Mar-21 26-Apr-21 26-May-21 Coronaviru��Update Islamic Republic of Iran| Coronavirus Disease 2019 (COVID-19) | 31 May 2021 2 Figure 3 - Number of Daily COVID-19 PCR Tests and Percentage of Positive Cases, 13 April 2020 - 31 May 2021 160,000 40% 140,000 35% 120,000 30% 100,000 25% 80,000 20% 60,000 15% rate Positivity No. -
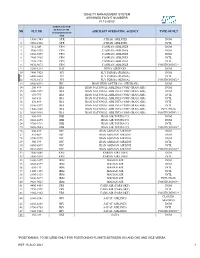
Quality Management System Assigned Flight Numbers Fi 13-03/00
QUALITY MANAGEMENT SYSTEM ASSIGNED FLIGHT NUMBERS FI 13-03/00 THREE LETTER DESIGNATOR NR FLT NR AIRCRAFT OPERATING AGENCY TYPE OF FLT ACCORDING DOC 8585 1 1900-1949 ATR ATRAK AIRLINES DOM 2 2902-2903 ATR ATRAK AIRLINES INTL 3 012-049 CPN CASPIAN AIRLINES DOM 4 4900-4939 CPN CASPIAN AIRLINES DOM 5 6900-6999 CPN CASPIAN AIRLINES DOM 6 7900-7969 CPN CASPIAN AIRLINES INTL 7 7970-7981 CPN CASPIAN AIRLINES INTL 8 9070-9073 CPN CASPIAN AIRLINES POSITIONING * 9 3200-3239 DAI DENA AIRWAYS DOM 10 7400-7425 FPI FLY PERSIA (PERSIA) DOM 11 6400-6403 FPI FLY PERSIA (PERSIA) INTL 12 9170-9173 FPI FLY PERSIA (PERSIA) POSITIONING * 13 8500-8599 IHC IRAN HELICOPTER CO. (HELIRAN) DOM 14 200-499 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) DOM 15 3300-3599 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) DOM 16 650-799 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) INTL 17 800-815 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) INTL 18 870-899 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) INTL 19 5300-5399 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) INTL 20 1500-1609 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) INTL(HAJJ) 21 9000-9003 IRA IRAN NATIONAL AIRLINES CORP.(IRAN AIR) POSITIONING * 22 900-999 IRB IRAN AIR TOURS CO. DOM 23 8000-8099 IRB IRAN AIR TOURS CO. DOM 24 9700-9719 IRB IRAN AIR TOURS CO. INTL 25 9080-9083 IRB IRAN AIR TOURS CO. POSITIONING * 26 600-649 IRC IRAN ASEMAN AIRLINE DOM 27 816-869 IRC IRAN ASEMAN AIRLINE DOM 28 3700-3999 IRC IRAN ASEMAN AIRLINE DOM 29 500-599 IRC IRAN ASEMAN AIRLINE INTL 30 6500-6599 IRC IRAN ASEMAN AIRLINE INTL 31 9010-9019 IRC IRAN ASEMAN -

IRAN AVIATION SUMMIT 2016 Tehran, 24-25 January
IRAN AVIATION SUMMIT 2016 Tehran, 24-25 January The first international aviation conference in Iran for nearly four decades More than 400 delegates and attendees – airlines, airports, aircraft and engine manufacturers, lessors, GDS and technology providers and suppliers – listened to aviation leaders and decision makers discussing the future of Iran’s aviation market. Some of the highlights were the announcement by Iran’s Deputy Minister for Roads & Urban Development of new aircraft orders from Airbus and ATR, Iran Air’s 10 year development plan, including making Tehran a major transit hub, and Turkish Airlines’ forecast of a doubling of tourism demand within two years. [More details inside] Visit the post-event website: centreforaviation.com/IRN2425 INFORM. CONNECT. INSPIRE. Highlights P14 Minister of Roads & Urban Development, Dr. Abbas Akhoundi Sponsors .................................P3 P16 Executive panel: Priority #1 sourcing and financing aircraft About CAPA - Centre for Aviation ...................................................... P4 CAPA Iran Aviation Summit International delegates ................... P5 Iran delegates .................................. P9 Gallery ..............................................P13 P18 Executive panel: Turkish Airlines, CEO, Dr Temel Kotil Day 1 agenda including CAPA News Briefs ...................P14 Networking dinner gallery ........................ P21 Day 2 agenda including CAPA News Briefs ......................... P22 CAPA Analysis ................................................P26 Contact -

AVIATION INDUSTRY in IRAN: INFRASTRUCTURE, CURRENT SYSTEM, and FUTURE PLANS and DEVELOPMENT Dana Khidhr [email protected]
Southern Illinois University Carbondale OpenSIUC Research Papers Graduate School Spring 2-9-2017 AVIATION INDUSTRY IN IRAN: INFRASTRUCTURE, CURRENT SYSTEM, AND FUTURE PLANS AND DEVELOPMENT Dana Khidhr [email protected] Follow this and additional works at: http://opensiuc.lib.siu.edu/gs_rp Recommended Citation Khidhr, Dana. "AVIATION INDUSTRY IN IRAN: INFRASTRUCTURE, CURRENT SYSTEM, AND FUTURE PLANS AND DEVELOPMENT." (Spring 2017). This Article is brought to you for free and open access by the Graduate School at OpenSIUC. It has been accepted for inclusion in Research Papers by an authorized administrator of OpenSIUC. For more information, please contact [email protected]. AVIATION INDUSTRY IN IRAN: INFRASTRUCTURE, CURRENT SYSTEM, AND FUTURE PLANS AND DEVELOPMENT by Dana Khidhr B.S., Salahaddin University, 2004 A Research Paper Submitted in Partial Fulfillment of the Requirements for the Master of Public Administration Department of Political Science in the Graduate School Southern Illinois University Carbondale May 2017 RESEARCH PAPER APPROVAL AVIATION INDUSTRY IN IRAN: INFRASTRUCTURE, CURRENT SYSTEM, AND FUTURE PLANS AND DEVELOPMENT By Dana Khidhr A Research Paper Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Public Administration in the field of Aviation Administration Approved by: Dr. David A NewMyer, Chair Dr. Stephanie Pink-Harper Gary Shafer Graduate School Southern Illinois University Carbondale Jan 30, 2017 AN ABSTRACT OF THE RESEARCH PAPER OF DANA KHIDHR, for the Master of Public Administration degree in PUBLIC ADMINISTRATION, presented on DECEMBER 16, 2016, at Southern Illinois University Carbondale. TITLE: AVIATION INDUSTRY IN IRAN: INFRASTRUCTURE, CURRENT SYSTEM, AND FUTURE PLANS AND DEVELOPMENT MAJOR PROFESSOR: Dr. -

Wikivoyage Iran March 2016 Contents
WikiVoyage Iran March 2016 Contents 1 Iran 1 1.1 Regions ................................................ 1 1.2 Cities ................................................. 1 1.3 Other destinations ........................................... 2 1.4 Understand .............................................. 2 1.4.1 People ............................................. 2 1.4.2 History ............................................ 2 1.4.3 Religion ............................................ 4 1.4.4 Climate ............................................ 4 1.4.5 Landscape ........................................... 4 1.5 Get in ................................................. 5 1.5.1 Visa .............................................. 5 1.5.2 By plane ............................................ 7 1.5.3 By train ............................................ 8 1.5.4 By car ............................................. 9 1.5.5 By bus ............................................. 9 1.5.6 By boat ............................................ 10 1.6 Get around ............................................... 10 1.6.1 By plane ............................................ 10 1.6.2 By bus ............................................. 11 1.6.3 By train ............................................ 11 1.6.4 By taxi ............................................ 11 1.6.5 By car ............................................. 12 1.7 Talk .................................................. 12 1.8 See ................................................... 12 1.8.1 Ancient cities -

Sam Chui Aviation & Travel
IRAN Airliners & Airports Flights and Sights PROVISIONAL ITINERARY & GENERAL INFORMATION Thursday, 18th – Thursday, 25th May, 2017 Our first ever aviation tour to the Islamic Republic of Iran! However, our itinerary has been organised by well-known aviation photographer Sam Chui who has arranged similar – and very successful and popular trips – over the last two year or so: we are in very capable hands. During our 8 days in Iran we will travel extensively around the country flying in various airliners and visiting a number of aviation sites. The itinerary also includes several sightseeing attractions and free time in Tehran. Please note airlines in Iran only release their domestic schedules one month in advance, so it is not possible to include actual domestic flight details now. However, as everything worked out fine with Sam’s earlier groups, we are very confident that w e will do all the internal flights as listed below . Tour highlights - including 10 DOMESTIC FLIGHTS - are: Fly from/to London’s Heathrow Airport on the scheduled A300-600 services of Iran Air Fly with Pouya Air on an Antonov An-74 Visit Saha Air (Iran Air Force) Simulator Flight Facility: optional ‘sim flights’ on B707, B747 & F27 Fly with Iran Aseman on the last B727-200 in passenger service – pictured above Fly to Shiraz with either Iran Air or Mahan Air MD-80 or A300 Fly to Isfahan by either Qeshem Air or Iran Air Avro RJ or Fokker-100 Fly to Ramzar on the coast of Caspian Sea by Aseman Airlines ATR-72: Optional light aircraft flights from Ramzar over the Caspian Sea Optional flight in VIP Falcon-20 Complimentary guided tour of Tehran Sightseeing/free time in Shiraz, Persepolis, Isfahan and Tehran We fly from London’s Heathrow Airport on the scheduled services of Iran Air and internally on various airlines as stated; stay in a 3* hotel located at Tehran Mehrabad Airport on a bed and breakfast basis; ground transfers are by de-luxe coach; the tour is accompanied by tour manager in addition to Sam Chui and local English-speaking guides. -

A Review of Iranian Aviation Industry: Victim of Sanctions Or Creation of Mismanagement?
A Review of Iranian Aviation Industry: Victim of Sanctions or Creation of Mismanagement? By: Ali Dadpay1 Abstract Motivated the present conditions of Iranian aviation industry this paper studies the history of Iranian aviation in the past three decades and its evolution from a public franchise to a mixed industry encompassing private, semi-private and public airlines. Exploring its different aspects, it demonstrates how commercial aviation has become accessible in many parts of country through ambitious projects to construct airports in many provinces and regions. It also shows that how increasing demand for domestic air travels has made commercial aviation industry self reliant and in no need to compete in international markets. It exhibits that how government interferes in the industry daily activities through pricing policies, fuel subsidies and granting licenses to fly different routes. Keyword: Iran, Aviation Industry, Commercial Airlines 1 Assistant professor of economics, School of Business, Clayton State University, 2000 Clayton State Blvd., Morrow GA 30260, visiting assistant professor, Graduate School of Management & Economics, Sharif University of Technology, Tehran, Iran. 1 Acknowledgement I would like to thank Mostafa Movafegh for his valuable assistance and persistence in following up the requests for data from Iran Civil Aviation Organization. I would like to acknowledge Vahid Nowshirvani for his insightful comments. Must be done: Define an index for accidents Create benchmarks for comparisons Study demand for: airlines, airports, routes, aircraft type Explaining the growth better 2 Introduction In 12 months that elapsed from March 2009 to March 2010 Iranian aviation industry experienced five accidents and three different heads of civil aviation.