Benlysta® (Belimumab) - Effective May 1, 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAFF-Neutralizing Interaction of Belimumab Related to Its Therapeutic Efficacy for Treating Systemic Lupus Erythematosus
ARTICLE DOI: 10.1038/s41467-018-03620-2 OPEN BAFF-neutralizing interaction of belimumab related to its therapeutic efficacy for treating systemic lupus erythematosus Woori Shin1, Hyun Tae Lee1, Heejin Lim1, Sang Hyung Lee1, Ji Young Son1, Jee Un Lee1, Ki-Young Yoo1, Seong Eon Ryu2, Jaejun Rhie1, Ju Yeon Lee1 & Yong-Seok Heo1 1234567890():,; BAFF, a member of the TNF superfamily, has been recognized as a good target for auto- immune diseases. Belimumab, an anti-BAFF monoclonal antibody, was approved by the FDA for use in treating systemic lupus erythematosus. However, the molecular basis of BAFF neutralization by belimumab remains unclear. Here our crystal structure of the BAFF–belimumab Fab complex shows the precise epitope and the BAFF-neutralizing mechanism of belimumab, and demonstrates that the therapeutic activity of belimumab involves not only antagonizing the BAFF–receptor interaction, but also disrupting the for- mation of the more active BAFF 60-mer to favor the induction of the less active BAFF trimer through interaction with the flap region of BAFF. In addition, the belimumab HCDR3 loop mimics the DxL(V/L) motif of BAFF receptors, thereby binding to BAFF in a similar manner as endogenous BAFF receptors. Our data thus provides insights for the design of new drugs targeting BAFF for the treatment of autoimmune diseases. 1 Department of Chemistry, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea. 2 Department of Bio Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 04763, Republic of Korea. These authors contributed equally: Woori Shin, Hyun Tae Lee, Heejin Lim, Sang Hyung Lee. -

Positioning Biologics in the Management of Moderate to Severe Crohn's Disease
REVIEW CURRENT OPINION Positioning biologics in the management of moderate to severe Crohn’s disease Jana G. Hashash and Fadi H. Mourad Purpose of review Since there is a lack of head-to-head randomized controlled trials, little direction is provided from guidelines on the positioning of biologics for the treatment of Crohn’s disease (CD). This review utilizes comparative effectiveness and safety results from real-world data and network meta-analyses to inform clinical practice for positioning of biological therapies in the treatment of moderate-to-severe CD. Recent findings We summarize the results of studies pertaining to the identification of predictors for response to biologics in CD. Recently published studies about the management of moderate-to-severe CD are discussed and a positioning algorithm is proposed for the therapeutic approach of these patients. Summary Different classes of biologics are comparable with regards to safety and almost similar in effectiveness in the management of CD. There are certain clinical scenarios in which one biologic is more effective than another. For instance, patients with a more aggressive disease phenotype such as fistulizing disease would benefit from infliximab over other biologics, whereas in older patients at a higher risk for infectious complications, it may be more appropriate to use ustekinumab or vedolizumab over the anti-tumor necrosis factor (TNF) agents. More data pertaining to identifying predictors of response to the different available therapies and head-to-head comparison trials are needed to personalize our therapeutic approach of CD patients. Keywords biologics, Crohn’s disease, moderate-to-severe, positioning INTRODUCTION The medical management of patients with CD To guide the choice of treatment in patients with has changed over recent years. -

Ocrevus (Ocrelizumab) Policy Number: C11250-A
Prior Authorization Criteria Ocrevus (ocrelizumab) Policy Number: C11250-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DUE BY OR BEFORE 8/1/2017 2/17/2021 4/26/2022 LAST P&T J CODE TYPE OF CRITERIA APPROVAL/VERSION J2350-injection,ocrelizumab, Q2 2021 RxPA 1mg 20200428C11250-A PRODUCTS AFFECTED: Ocrevus (ocrelizumab) DRUG CLASS: Multiple Sclerosis Agents - Monoclonal Antibodies ROUTE OF ADMINISTRATION: Intravenous PLACE OF SERVICE: Specialty Pharmacy or Buy and Bill The recommendation is that medications in this policy will be for pharmacy benefit coverage and the IV infusion products administered in a place of service that is a non-hospital facility-based location (i.e., home infusion provider, provider’s office, free-standing ambulatory infusion center) AVAILABLE DOSAGE FORMS: Ocrevus SOLN 300MG/10ML FDA-APPROVED USES: Indicated for the treatment of: • Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing- remitting disease, and active secondary progressive disease, in adults • Primary progressive MS, in adults COMPENDIAL APPROVED OFF-LABELED USES: None COVERAGE CRITERIA: INITIAL AUTHORIZATION DIAGNOSIS: Multiple Sclerosis REQUIRED MEDICAL INFORMATION: A. RELAPSING FORMS OF MULTIPLE SCLEROSIS: 1. Documentation of a definitive diagnosis of a relapsing form of multiple sclerosis as defined by the McDonald criteria (see Appendix), including: Relapsing- remitting multiple sclerosis [RRMS], secondary-progressive multiple sclerosis [SPMS] with relapses, and progressive- relapsing multiple sclerosis [PRMS] or First clinical episode with MRI features consistent with multiple sclerosis Molina Healthcare, Inc. confidential and proprietary © 2021 This document contains confidential and proprietary information of Molina Healthcare and cannot be reproduced, distributed, or printed without written permission from Molina Healthcare. -

Xolair/Omalizumab
Therapeutic/Humanized Antibodies ELISA Kits ‘Humanized antibodies” are ‘Animal Antibodies’ that have been modified by recombinant DNA-technology to reduce the overall content of the animal- portion of immunoglobulin so as to increase acceptance by humans or minimize ‘rejection’. By analogy, if the hands of a mouse is actually responsible for grabbing things then ‘humanized-mouse’ will only contain the ‘mouse hands’ and the rest of the body will be human. Since the size of the IgGs is similar in mouse and human, the size of the native mouse IgG and the ‘humanized IgG’ does not significantly change. Antigen recognition property of an antibody actually resides in small portion of the IgG molecule called the ‘antigen binding site or Fab’. Therefore, humanized antibodies contain the minimal portion from the Fab or the epitopes necessary for antigen binding. The process of "humanization of antibodies" is usually applied to animal (mouse) derived monoclonal antibodies for therapeutic use in humans (for example, antibodies developed as anti-cancer drugs). Xolair (Anti-IgE) is an example of humanized IgG. The portion of the mouse IgG that remains in the ‘humanized IgG’ may be recognized as foreign by humans and may result into the generation of “Human Anti-Drug Antibodies (HADA). The presence of anti-Drug antibody (e.g., Human Anti-Rituximab IgG) that may limit the long-term usage the humanized antibody (Rituximab). Not all monoclonal antibodies designed for human therapeutic use need be humanized since many therapies are short-term. The International Nonproprietary Names of humanized antibodies end in “-zumab”, as in “omalizumab”. Humanized antibodies are distinct from chimeric or fusion antibodies. -

Exploring the Association Between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: a Vigibase Study
Drug Safety https://doi.org/10.1007/s40264-018-00789-9 ORIGINAL RESEARCH ARTICLE Exploring the Association between Monoclonal Antibodies and Depression and Suicidal Ideation and Behavior: A VigiBase Study Lotte A. Minnema1,2 · Thijs J. Giezen2,3 · Patrick C. Souverein1 · Toine C. G. Egberts1,4 · Hubert G. M. Leufkens1 · Helga Gardarsdottir1,4,5 © The Author(s) 2019 Abstract Introduction Several monoclonal antibodies (mAbs) have been linked to neuropsychiatric adverse efects in patients, includ- ing depression and suicidal ideation and behavior. Objective The aim of this study was to quantify and characterize spontaneously reported adverse drug reactions (ADRs) of depression and suicidal ideation and behavior related to mAb users, and to explore a possible association with their mecha- nism of action. Methods We included mAb ADRs that were reported in VigiBase, and identifed those related to depression and suicidal ideation and behavior. Reporting odds ratios (RORs) were estimated for each mAb (bevacizumab as the reference) and according to their infuence on the immune system (not directly targeting [reference], stimulating, or suppressing). Those suppressing the immune system were further divided into their intended indication (auto-immune diseases, cancer). Results Overall, 2,924,319 ADRs for 44 mAbs were included; 9455 ADRs were related to depression and 1770 were related to suicidal ideation and behavior. The association was strongest for natalizumab and belimumab, both for depression (ROR 5.7, 95% confdence interval [CI] 5.0–6.4; and ROR 5.1, 95% CI 4.2–6.2) and suicidal ideation and behavior (ROR 12.0, 95% CI 7.9–18.3; and ROR 20.2, 95% CI 12.4–33.0). -

Cutaneous Adverse Effects of Biologic Medications
REVIEW CME MOC Selena R. Pasadyn, BA Daniel Knabel, MD Anthony P. Fernandez, MD, PhD Christine B. Warren, MD, MS Cleveland Clinic Lerner College Department of Pathology Co-Medical Director of Continuing Medical Education; Department of Dermatology, Cleveland Clinic; of Medicine of Case Western and Department of Dermatology, W.D. Steck Chair of Clinical Dermatology; Director of Clinical Assistant Professor, Cleveland Clinic Reserve University, Cleveland, OH Cleveland Clinic Medical and Inpatient Dermatology; Departments of Lerner College of Medicine of Case Western Dermatology and Pathology, Cleveland Clinic; Assistant Reserve University, Cleveland, OH Clinical Professor, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH Cutaneous adverse effects of biologic medications ABSTRACT iologic therapy encompasses an expo- B nentially expanding arena of medicine. Biologic therapies have become widely used but often As the name implies, biologic therapies are de- cause cutaneous adverse effects. The authors discuss the rived from living organisms and consist largely cutaneous adverse effects of tumor necrosis factor (TNF) of proteins, sugars, and nucleic acids. A clas- alpha inhibitors, epidermal growth factor receptor (EGFR) sic example of an early biologic medication is inhibitors, small-molecule tyrosine kinase inhibitors insulin. These therapies have revolutionized (TKIs), and cell surface-targeted monoclonal antibodies, medicine and offer targeted therapy for an including how to manage these reactions -

Curriculum Vitae: Daniel J
CURRICULUM VITAE: DANIEL J. WALLACE, M.D., F.A.C.P., M.A.C.R. Up to date as of January 1, 2019 Personal: Address: 8750 Wilshire Blvd, Suite 350 Beverly Hills, CA 90211 Phone: (310) 652-0010 FAX: (310) 360-6219 E mail: [email protected] Education: University of Southern California, 2/67-6/70, BA Medicine, 1971. University of Southern California, 9/70-6/74, M.D, 1974. Postgraduate Training: 7/74-6/75 Medical Intern, Rhode Island (Brown University) Hospital, Providence, RI. 7/75-6/77 Medical Resident, Cedars-Sinai Medical Center, Los Angeles, CA. 7/77-6/79 Rheumatology Fellow, UCLA School of Medicine, Los Angeles, CA. Medical Boards and Licensure: Diplomate, National Board of Medical Examiners, 1975. Board Certified, American Board of Internal Medicine, 1978. Board Certified, Rheumatology subspecialty, 1982. California License: #G-30533. Present Appointments: Medical Director, Wallace Rheumatic Study Center Attune Health Affiliate, Beverly Hills, CA 90211 Attending Physician, Cedars-Sinai Medical Center, Los Angeles, 1979- Clinical Professor of Medicine, David Geffen School of Medicine at UCLA, 1995- Professor of Medicine, Cedars-Sinai Medical Center, 2012- Expert Reviewer, Medical Board of California, 2007- Associate Director, Rheumatology Fellowship Program, Cedars-Sinai Medical Center, 2010- Board of Governors, Cedars-Sinai Medical Center, 2016- Member, Medical Policy Committee, United Rheumatology, 2017- Honorary Appointments: Fellow, American College of Physicians (FACP) Fellow, American College of Rheumatology (FACR) -

B.C. Pharmacare Drug Information Sheet for Vedolizumab (Entyvio)
The drug below is being considered for possible coverage under the B.C. PharmaCare program. PharmaCare is a government-funded drug plan that helps British Columbians with the cost of eligible prescription drugs and specific medical supplies. For more information on PharmaCare, visit Ministry of Health - PharmaCare. PharmaCare reviews each drug for treating a specific illness or medical condition (known as an “indication”). If a decision is made to cover the drug, it will be only for that illness or condition. In some cases, PharmaCare may cover a drug only for people who have the illness or condition and have not responded to other drugs used to treat that illness or condition. For more information on PharmaCare’s drug coverage review process, see the last page of this information sheet. Information about the drug Generic name (scientific name) vedolizumab Brand name Entyvio® Manufacturer Takeda Canada Inc. Indication Entyvio is indicated for the treatment of moderately to severely active Crohn’s disease in adult patients. Has the drug been reviewed by Yes the Common Drug For more information about the CDR’s review of vedolizumab (Entyvio®), you can Review (CDR)? Search the CDR Reports. (see the note below this table.) Public input start date Friday, November 13, 2020 Public input closing date Friday, December 11, 2020 AT MIDNIGHT How is the drug taken? Entyvio is administered by subcutaneous injection (into the skin). How often is the drug taken? Entyvio is administered every two weeks, following at least two intravenous infusions (into the vein). Ministry of Health Pharmaceutical Services Division Page 1 of 4 BC PharmaCare Drug Information — vedolizumab (Entyvio®) continued… Information about the drug General drug and/or drug Crohn’s disease is a chronic inflammatory disease of the gastrointestinal tract, which study information most commonly affects the small intestine, beginning of large intestine, and rectum. -

Risk Assessment and Risk Mitigation Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125476Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology Office of Medication Error Prevention and Risk Management Final Risk Evaluation and Mitigation Strategy (REMS) Review Date: May 7, 2014 Reviewer(s): George Neyarapally, Pharm.D., M.P.H. Division of Risk Management (DRISK) Team Leader: Reema Mehta, Pharm.D., M.P.H. DRISK Division Director: Claudia Manzo, Pharm.D. DRISK Drug Name(s): Entyvio (vedolizumab) Therapeutic Class: Lymphocyte α4β7 integrin receptor antagonist Dosage and Route: Solution for infusion Application Type/Number: BLA 125476, BLA 125507 Applicant/Sponsor: Takeda Pharmaceuticals OSE RCM #: 2013-1641 *** This document contains proprietary and confidential information that should not be released to the public. *** 1 Reference ID: 3502774 CONTENTS 1 INTRODUCTION ....................................................................................................... 1 1.1 Product Background............................................................................................ 1 1.2 Disease Background............................................................................................ 2 1.3 Regulatory History .............................................................................................. 2 2 MATERIALS REVIEWED ....................................................................................... -

Datasheet: HCA295 Product Details
Datasheet: HCA295 Description: HUMAN ANTI VEDOLIZUMAB Specificity: VEDOLIZUMAB Other names: Entyvio Format: Purified Product Type: Monoclonal Antibody Clone: AbD30139_hIgG1 Isotype: IgG1 Quantity: 0.1 mg Product Details Applications This product has been reported to work in the following applications. This information is derived from testing within our laboratories, peer-reviewed publications or personal communications from the originators. Please refer to references indicated for further information. For general protocol recommendations, please visit www.bio-rad-antibodies.com/protocols. Yes No Not Determined Suggested Dilution ELISA Where this product has not been tested for use in a particular technique this does not necessarily exclude its use in such procedures. Suggested working dilutions are given as a guide only. It is recommended that the user titrates the product for use in their own system using appropriate negative/positive controls. Product Form Human IgG1 antibody (lambda light chain) selected from the HuCAL® phage display library and expressed in a human cell line. This antibody is supplied as a liquid. Preparation Purified IgG prepared by affinity chromatography on Protein A Buffer Solution Phosphate buffered saline Preservative 0.01% Thiomersal Stabilisers Approx. Protein Antibody concentration 0.5 mg/ml Concentrations Immunogen Vedolizumab Specificity Human Anti-Vedolizumab Antibody, AbD30139_hIgG1 is a paratope specific, inhibitory anti-idiotypic antibody that specifically recognizes the monoclonal antibody drug vedolizumab. The antibody does not recognize free recombinant human α4β7 integrin or vedolizumab in complex with α4β7 integrin and can be used to measure free vedolizumab levels in serum from patients. Clone AbD30139_hIgG1 is a fully human recombinant monoclonal antibody with IgG1 isotype and is suitable as a reference standard in an anti-drug antibody (ADA) assay. -
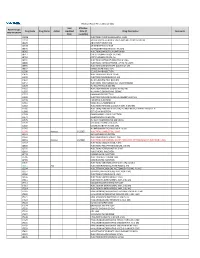
2021 Prior Authorization List Part B Appendix a (PDF)
Medicare Part B PA List Effective 2021 Last Effective Part B Drugs: Drug Code Drug Name Action Updated Date (if Drug Description Comments STEP THERAPY Date available) C9050 INJECTION, EMAPALUMAB-LZSG, 1 MG C9122 MOMETASONE FUROATE SINUS IMPLANT 10 MCG SINUVA J0129 ABATACEPT INJECTION J0178 AFLIBERCEPT INJECTION J0570 BUPRENORPHINE IMPLANT 74.2MG J0585 INJECTION,ONABOTULINUMTOXINA J0717 CERTOLIZUMAB PEGOL INJ 1MG J0718 CERTOLIZUMAB PEGOL INJ J0791 INJECTION CRIZANLIZUMAB-TMCA 5 MG J0800 INJECTION, CORTICOTROPIN, UP TO 40 UNITS J0896 INJECTION LUSPATERCEPT-AAMT 0.25 MG J0897 DENOSUMAB INJECTION J1300 ECULIZUMAB INJECTION J1428 INJECTION ETEPLIRSEN 10 MG J1429 INJECTION GOLODIRSEN 10 MG J1442 INJ FILGRASTIM EXCL BIOSIMIL J1447 INJECTION, TBO-FILGRASTIM, 1 MICROGRAM J1459 INJ IVIG PRIVIGEN 500 MG J1555 INJECTION IMMUNE GLOBULIN 100 MG J1556 INJ, IMM GLOB BIVIGAM, 500MG J1557 GAMMAPLEX INJECTION J1558 INJECTION IMMUNE GLOBULIN XEMBIFY 100 MG J1559 HIZENTRA INJECTION J1561 GAMUNEX-C/GAMMAKED J1562 INJECTION; IMMUNE GLOBULIN 10%, 5 GRAMS J1566 INJECTION, IMMUNE GLOBULIN, INTRAVENOUS, LYOPHILIZED (E.G. P J1568 OCTAGAM INJECTION J1569 GAMMAGARD LIQUID INJECTION J1572 FLEBOGAMMA INJECTION J1575 INJ IG/HYALURONIDASE 100 MG IG J1599 IVIG NON-LYOPHILIZED, NOS J1602 GOLIMUMAB FOR IV USE 1MG J1745 INJ INFLIXIMAB EXCL BIOSIMILR 10 MG J1930 Remove 1/1/2021 INJECTION, LANREOTIDE, 1 MG J2323 NATALIZUMAB INJECTION J2350 INJECTION OCRELIZUMAB 1 MG J2353 Remove 1/1/2021 INJECTION, OCTREOTIDE, DEPOT FORM FOR INTRAMUSCULAR INJECTION, 1 MG J2357 INJECTION, OMALIZUMAB, -

Cimzia (Certolizumab Pegol) AHM
Cimzia (Certolizumab Pegol) AHM Clinical Indications • Cimzia (Certolizumab Pegol) is considered medically necessary for adult members 18 years of age or older with moderately-to-severely active disease when ALL of the following conditions are met o Moderately-to-severely active Crohn's disease as manifested by 1 or more of the following . Diarrhea . Abdominal pain . Bleeding . Weight loss . Perianal disease . Internal fistulae . Intestinal obstruction . Megacolon . Extra-intestinal manifestations: arthritis or spondylitis o Crohn's disease has remained active despite treatment with 1 or more of the following . Corticosteroids . 6-mercaptopurine/azathioprine • Certollizumab pegol (see note) is considered medically necessary for persons with active psoriatic arthritis who meet criteria in Psoriasis and Psoriatic Arthritis: Biological Therapies. • Cimzia, (Certolizumab Pegol), alone or in combination with methotrexate (MTX), is considered medically necessary for the treatment of adult members 18 years of age or older with moderately-to-severely active rheumatoid arthritis (RA). • Cimzia (Certolizumab pegol is considered medically necessary for reducing signs and symptoms of members with active ankylosing spondylitis who have an inadequate response to 2 or more NSAIDs. • Cimzia (Certolizumab Pegol) is considered investigational for all other indications (e.g.,ocular inflammation/uveitis; not an all-inclusive list) because its effectiveness for indications other than the ones listed above has not been established. Notes • There are several brands of targeted immune modulators on the market. There is a lack of reliable evidence that any one brand of targeted immune modulator is superior to other brands for medically necessary indications. Enbrel (etanercept), Humira (adalimumab), Remicade (infliximab), Simponi (golimumab), Simponi Aria (golimumab intravneous), and Stelara (ustekinumab) brands of targeted immune modulators ("least cost brands of targeted immune modulators") are less costly to the plan.