Fleas (Siphonaptera) of Mammals and Birds in the Great Caucasus B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -
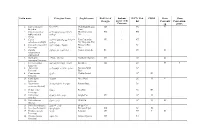
Red List of Endemic IUCN Red CITES Bern Bonn Georgia Species of the List Conventi Convention Caucasus on (CMS) 1
Latin name Georgian Name English name Red List of Endemic IUCN Red CITES Bern Bonn Georgia species of the list Conventi Convention Caucasus on (CMS) 1. Capra aegagrus niamori Wild Goat, Bezoar CR VU II Erxleben. Goat 2. Capra caucasica dasavleTkavkasiuri West Caucasian EN + EN Güldenstädt & jixvi Tur Pallas. 3. Capra aRmosavleTkavkasiuri East Caucasian VU + NT cylindricornis Blyth. jixvi Tur, Dagestan Tur 4. Capreolus capreolus evropuli Sveli European Roe LC Linnaeus. Deer 5. Gazella qurciki, jeirani Goitered Gazelle RE VU II subgutturosa Güldenstädt. 6. Rupicapra arCvi, fsiti Northern Chamois EN LC II rupicapra Linnaeus. 7. Cervus elaphus keTilSobili iremi Red Deer CR LC II I Linnaeus. 8. Sus scrofa gareuli Rori, taxi Eurasian Wild LC Linnaeus. Boar 9. Canis aureus tura Golden Jackal LC III Linnaeus. 10. Canis lupus mgeli Grey Wolf LC II II Linnaeus. 11. Nyctereutes enotisebri ZaRli Racoon Dog LC procyonoides Gray. 12. Vulpes vulpes mela Red Fox LC III Linnaeus. 13. Felis chaus lelianis kata Jungle Cat VU LC II Schreber. 14. Felis silvestris tyis kata Wild Cat LC II II Shreber. 15. Felis libyca Forster. velis kata Steppe Cat 16. Lynx lynx Linnaeus. focxveri Eurasian Lynx CR LC II 17. Panthera pardus jiqi Leopard CR NT I II Linnaeus. 18. Hyaena hyaena afTari Striped Hyaena CR NT Linnaeus. 19. Lutra lutra wavi Eurasian Otter, VU NT I II Linnaeus. Common Otter 20. Martes foina kldis kverna Stone Marten, LC III Erxleben. Beech Marten 21. Martes martes tyis kverna European Pine LC Linnaeus. Marten 22. Meles meles maCvi Eurasian Badger LC Linnaeus. 23. Mustela lutreola waula European Mink EN II Linnaeus. -

Ewa Żurawska-Seta
Tom 59 2010 Numer 1–2 (286–287) Strony 111–123 Ewa Żurawska-sEta Katedra Zoologii Uniwersytet Technologiczno-Przyrodniczy A. Kordeckiego 20, 85-225 Bydgoszcz E-mail: [email protected] kretowate talpidae — ROZMieSZCZeNie oraZ klaSyfikaCja w świetle badań geNetycznyCh i MorfologicznyCh wStęp podział systematyczny ssaków podlega zaproponowanej przez wilson’a i rEEdEr’a ciągłym zmianom, głównie za sprawą dyna- (2005) wyodrębnione zostały nowe rzędy: micznie rozwijających się w ostatnich latach erinaceomorpha, do którego zaliczono je- badań genetycznych. w klasyfikacji owado- żowate erinaceidae oraz Soricomorpha, w żernych insectivora zaszły istotne zmiany. którym znalazły się kretowate talpidae i ry- według tradycyjnej klasyfikacji fenetycznej jówkowate Soricidae. do ostatniego z wymie- kret Talpa europaea linnaeus, 1758, zali- nionych rzędów zaliczono ponadto almiko- czany był do rodziny kretowatych talpidae wate Solenodontidae oraz wymarłą rodzinę i rzędu owadożernych insectivora. do tego Nesophontidae (wcześniej obie te rodziny samego rzędu należały również występujące również zaliczano do insectivora), z zastrze- w polsce jeże i ryjówki. według klasyfikacji żeniem, że oczekiwane są dalsze zmiany. klaSyfikaCja kretowatyCh talpidae rodzina talpidae obejmuje 3 podrodziny: świetnym pływakiem, ponieważ większość Scalopinae, talpinae i Uropsilinae. podrodzi- pożywienia zdobywa na powierzchni wody na Scalopinae podzielona jest na 2 plemiona, oraz w toni wodnej. jest do tego doskona- 5 rodzajów i 7 gatunków (tabela 1). krety z le przystosowany, ponieważ posiada błony tej podrodziny często nazywane są „kretami z pławne między palcami kończyn tylnych. Nowego świata”, ponieważ większość gatun- Ma również długi ogon, stanowiący 75–81% ków występuje w Stanach Zjednoczonych, długości całego ciała, który spełnia rolę ma- północnym Meksyku i w południowej części gazynu tłuszczu, wykorzystywanego głównie kanady. -

Activity of the European Mole Talpa Europaea (Talpidae, Insectivora) in Its Burrows in the Republic of Mordovia
FORESTRY IDEAS, 2021, vol. 27, No 1 (61): 59–67 ACTIVITY OF THE EUROPEAN MOLE TALPA EUROPAEA (TALPIDAE, INSECTIVORA) IN ITS BURROWS IN THE REPUBLIC OF MORDOVIA Alexey Andreychev Department of Zoology, National Research Mordovia State University, Saransk 430005, Russia. E-mail: [email protected] Received: 16 February 2021 Accepted: 18 April 2021 Abstract A new method of studying the activity of European mole Talpa europaea (Linnaeus, 1758) with use of digital portable voice recorders is developed. European mole demonstrates polyphasic activity pattern – three peaks of activity alternate three peaks of relative rest. Moles are found to have activity peaks from 23:00 to 3:00 h, from 6:00 to 9:00 h and from 15:00 to 18:00 h, and three periods of rest: from 3:00 to 6:00 h, from 9:00 to 15:00 h and from 18:00 to 23:00 h. Mole’s rest is relative since animals show low activity during periods of rest. Average daily interval between mole passes is 2.5 h. Duration of audibility of continuous single European mole pass by the mi- crophone varies from 11 to 120 seconds. On average, it is 37.5 seconds. Key words: animals, daily activity, day-night activity, voice recorder. Introduction where light factor is essential (Shilov 2001). Activity of many mammals is in- In many countries, the European mole Tal- vestigated under various conditions of the pa europaea (Linnaeus, 1758) is an object light regime. For underground animals, of constant modern research (Komarnicki especially for the different mole species, 2000, Prochel 2006, Kang et al. -

Rodent Societies: an Ecological & Evolutionary Perspective
Chapter 16 Neural Regulation of Social Behavior in Rodents J. Thomas Curtis, Yan Liu, Brandon J. Aragona, and Zuoxin Wang ocial behavior arises from a complex interplay since behaviors such as aggression likely derive from the of numerous and often-competing sensory stimuli, the mating system. S physiological and motivational states of the partici- Rodents are a diverse group of creatures that inhabit a pants, and the ages and genders of the individuals involved. wide variety of ecological niches. As might be expected of Overlying the internal responses to a social encounter are such diversity, rodents display a wide range of mating sys- a variety of external factors, such as the context in which tems. Males and females of many species often have differ- the encounter occurs, the time of year, environmental con- ent mating strategies (Waterman, chap. 3, Solomon and ditions, and the outcomes of previous social interactions. Keane, chap. 4, this volume). However, some environmental To further complicate the situation, each individual in a so- conditions require extensive cooperation between the sexes cial encounter must be able to adjust its own actions de- for reproductive success (Kleiman 1977). In these cases, the pending on the responses of other animals. Given such com- mating strategies of the two sexes may converge and a plexity, a detailed understanding of the neural basis of social monogamous mating system may arise. Only about 3% of behavior would seem next to impossible. Nonetheless, con- mammalian species have been categorized as being monog- siderable progress has been made. By examining individual amous (Kleiman 1977). -

What Should We Call the Levant Mole? Unravelling the Systematics and Demography of Talpa Levantis Thomas, 1906 Sensu Lato (Mammalia: Talpidae)
University of Plymouth PEARL https://pearl.plymouth.ac.uk Faculty of Science and Engineering School of Biological and Marine Sciences 2020-03-02 What should we call the Levant mole? Unravelling the systematics and demography of Talpa levantis Thomas, 1906 sensu lato (Mammalia: Talpidae) Demirtas, S http://hdl.handle.net/10026.1/15424 10.1007/s42991-020-00010-4 Mammalian Biology Elsevier All content in PEARL is protected by copyright law. Author manuscripts are made available in accordance with publisher policies. Please cite only the published version using the details provided on the item record or document. In the absence of an open licence (e.g. Creative Commons), permissions for further reuse of content should be sought from the publisher or author. 1 What should we call the Levant mole? Unravelling the systematics and demography of 2 Talpa levantis Thomas, 1906 sensu lato (Mammalia: Talpidae). 3 4 Sadik Demirtaşa, Metin Silsüpüra, Jeremy B. Searleb, David Biltonc,d, İslam Gündüza,* 5 6 aDepartment of Biology, Faculty of Arts and Sciences, Ondokuz Mayis University, Samsun, 7 Turkey. 8 bDepartment of Ecology and Evolutionary Biology, Cornell University, Ithaca, NY, 14853- 9 2701, USA. 10 cMarine Biology and Ecology Research Centre, School of Biological and Marine Sciences, 11 University of Plymouth, Plymouth PL4 8AA, Devon, UK. 12 dDepartment of Zoology, University of Johannesburg, PO Box 524, Auckland Park, 13 Johannesburg 2006, Republic of South Africa 14 15 *Corresponding author. E-mail: [email protected] 16 1 17 Abstract 18 19 Turkey hosts five of the eleven species of Talpa described to date, Anatolia in particular 20 appearing to be an important centre of diversity for this genus. -

A-Nadachowski.Vp:Corelventura
Acta zoologica cracoviensia, 50A(1-2): 67-72, Kraków, 31 May, 2007 The taxonomic status of Schelkovnikov’s Pine Vole Microtus schelkovnikovi (Rodentia, Mammalia) Adam NADACHOWSKI Received: 11 March, 2007 Accepted: 20 April, 2007 NADACHOWSKI A. 2007. The taxonomic status of Schelkovnikov’s Pine Vole Microtus schelkovnikovi (Rodentia, Mammalia). Acta zoologica cracoviensia, 50A(1-2): 67-72. Abstract. A comparison of morphological and karyological traits as well as an analysis of ecological preferences and the distribution pattern support the opinion that Microtus schelkovnikovi does not belong to subgenus Terricola and is the sole member of its own taxonomic species group. Hyrcanicola subgen. nov. comprises a single species Microtus (Hyrcanicola) schelkovnikovi, an endemic and relict form, inhabiting the Hyrcanian broad-leaved forest zone of Azerbaijan and Iran. Key-words: Systematics, new taxon, voles, Hyrcanian forests. Adam NADACHOWSKI, Institute of Systematics and Evolution of Animals, Polish Acad- emy of Sciences, S³awkowska 17, 31-016 Kraków, Poland. E-mail: [email protected] I. INTRODUCTION Schelkovnikov’s Pine Vole (Microtus schelkovnikovi SATUNIN, 1907) is one of the most enig- matic voles represented in natural history collections by only 45-50 specimens globally. This spe- cies was described by K. A. SATUNIN, on the basis of a single male specimen collected by A. B. SHELKOVNIKOV, July 6, 1906 near the village of Dzhi, in Azerbaijan (SATUNIN 1907). Next, 14 specimens from Azerbaijan, were found by KH.M.ALEKPEROV 50 years later in 1956 and 1957 (ALEKPEROV 1959). ELLERMAN (1948) described a new subspecies of Pine Vole Pitymys subterra- neus dorothea, on the basis of 3 females, collected by G. -

Terrestrial Ecology
Chapter 11: Terrestrial Ecology URS-EIA-REP-204635 Table of Contents 11 Terrestrial Ecology ................................................................................... 11-1 11.1 Introduction ...................................................................................................... 11-1 11.2 Scoping ............................................................................................................ 11-1 11.2.1 ENVIID ................................................................................................ 11-2 11.2.2 Stakeholder Engagement ...................................................................... 11-2 11.2.3 Analysis of Alternatives ......................................................................... 11-4 11.3 Spatial and Temporal Boundaries ........................................................................ 11-4 11.3.1 Spatial Boundaries ................................................................................ 11-4 11.3.2 Temporal Boundaries .......................................................................... 11-11 11.4 Baseline Data .................................................................................................. 11-11 11.4.1 Introduction ....................................................................................... 11-11 11.4.2 Secondary Data .................................................................................. 11-11 11.4.3 Data Gaps .......................................................................................... 11-14 -

The Mammals of the Caucasus
planifrons Falc. and Equus stenonis Cocchi have been identified. The breccia is underlain by a layer of doleritic lava and is covered by lacustrine sands and clays. The lake sediments are overlain by a layer of dolorite (Zaridze and Tatrishvili, 1948). Thus, in that area, the mammals lived and died during a period when the volcanos in the Lesser Caucasus were dornnant. The next transgression in the Caspian ->- N Basin, a somewhat smaller one, is known ^.AA.>.>J>-Jn,*^f^^v-»L*.-.->4-W-vn-Wtv^ as the Apsheron sea. ;'<,•.•''' OS The Kura bay of the Apsheron sea OB reached the longitude of Kirovabad. The :o:.\-:e:.--i Terek bay was temporarily connected with the Euxinic basin by a strait in the Manych area. The sea reached the latitude of Sarepta and [Lake] Inder in the north. •'•p.;-. .- •«.•.. e". ;..•.*• o*. •.•.; ''•',' 45 o'. • o". ' '•'o'.'- o." ; •.b..'i^-'.'-o'".^•.•<>.• The climate and landforms of the 4>- b-fi.: Caucasus in Apsheron time probably remained the same as in the Akchagyl, and the volcanic activity was of about the same c?o intensity. Torrential mudflows, caused by heavy rains, carried volumes of gravel and boulders from the mountains (Kudryavtsev, 1933); these boulders can 68 'S::^^^^ now be seen on the Kakhetia Plain. d - 5 The land vegetation known from the Apsheron deposits in the Shiraki Steppe consisted of spruce (Picea orientalis) and a number of Recent forms: beech, oak, aspen, apple, willow, filbert, Turkish filbert, walnut (Juglans regia), zelkova, honeysuckle; and Hyrcanian forms: f < oak (Quercus castanei folia), alder (Alnus subcordata), maple (Acer ibericum) (Palibin, 1936). -

National Biodiversity Strategy and Action Plan of Georgia
Biodiversity Strategy and Action Plan - Georgia – Tbilisi, 2005 Foreword Georgia signed the Convention on Biological Diversity in 1994, thus accepting responsibility to safeguard the nation’s rich diversity of plant, animal, and microbial life, to begin using biological resources in sustainable way, and to ensure equitable sharing of benefits from biodiversity. Later the country joined other conventions including the Convention on Climate Change, the Ramsar Convention on Wetlands, CITES and the Bonn Convention. As a signatory to these important international environmental treaties, Georgia enters the world scene with the potential for joining the most advanced nations in the field of environmental protection. At the present moment of transition, Georgia has a unique opportunity to use the early experiences of other countries, and avoid irreversible changes in the quality of its environment. The national legislation on environmental protection adopted over the past few years provides an adequate legal basis for this, although further elaboration and reinforcement of the existing legislation is needed. With the Ministry of Environment being currently reorganised and assuming broader responsibilities, Georgia’s institutional arrangements for environmental protection already has the necessary structure for improving the quality of the environment throughout the country. The role of non-governmental groups has been very important in resolving problems related to nature conservation. Georgia has shown an excellent example of co-operation between governmental and non-governmental organizations in the field of environment, and particularly in the field of biodiversity conservation. After signing the Convention on Biological Diversity, the Georgian Government immediately acted to develop a Biodiversity Country Study, in partnership with UNEP, and implemented by NACRES, a local conservation organisation. -

A New Species of Voles, Microtus Elbeyli Sp. Nov., from Turkey with Taxonomic Overview of Social Voles Distributed in Southeastern Anatolia
Turkish Journal of Zoology Turk J Zool (2016) 40: 73-79 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-1404-19 A new species of voles, Microtus elbeyli sp. nov., from Turkey with taxonomic overview of social voles distributed in southeastern Anatolia 1 1 2 Nuri YİĞİT , Ercüment ÇOLAK , Mustafa SÖZEN 1 Department of Biology, Faculty of Science, Ankara University, Ankara, Turkey 2 Department of Biology, Faculty of Arts and Sciences, Bülent Ecevit University, Zonguldak, Turkey Received: 10.04.2015 Accepted/Published Online: 05.09.2015 Final Version: 01.01.2016 Abstract: There are twelve Microtus species in Turkey and two of them are endemic to the steppic central Anatolian plateau. In this study, previously collected specimens that were recorded as Microtus irani from southeastern Turkey were reevaluated by karyologically comparing different species distributed throughout southeastern Turkey. The taxonomic status of this species was raised to a new species, Microtus elbeyli sp. nov., which has dark ochreous dorsal color, agrestis morphotype in M2, and 2n = 46, NF = 50, NFa = 46 karyotype. The new species described here raises the total number of Microtus species in Turkey to 13 and endemic vole species in Anatolia to three. Key words: Microtus elbeyli, taxonomy, morphology, distribution, new species, Turkey 1. Introduction record of M. irani by Çolak et al. (1997), another diploid Voles in the genus Microtus were represented by 12 species chromosome number (2n = 60) for the same species was in Turkey (Yiğit et al., 2006b; Kryštufek and Vohralík, reported from southern Turkey by Kryštufek et al. (2009). -

2006 Isbn 99940-58-55-X
AN ECOREGIONAL CONSERVATION PLAN FOR THE CAUCASUSAN ECOREGIONAL CONSERVATION PLAN FOR THE CAUCASUS Second Edition May 2006 ISBN 99940-58-55-X Design and printing Contour Ltd 8, Kargareteli street, Tbilisi 0164, Georgia May, 2006 Coordinated by: In collaboration with: With the technical support of: Assisted by experts and contributors: ARMENIA MAMMEDOVA, S. NAKHUTSRISHVILI, G. POPOVICHEV, V. AGAMYAN, L. MUKHTAROV, I. NINUA, N. PTICHNIKOV, A. AGASYAN, A. NAJAFOV, A. SERGEEVA, J. BELANOVSKAYA, E. AKOPYAN, S. ORUJEV, Ad. SIKHARULIDZE, Z. SALPAGAROV, A. AMBARTSUMYAN, A. ORUJEV, Al. SOPADZE, G. SHESTAKOV, A ARZUMANYAN, G. RAKHMATULINA, I. TARKHNISHVILI, D. SKOROBOGACH, J. BALYAN, L. RZAEV, R. TOLORDAVA, K. SPIRIDONOV, V. DANYELYAN, T. SATTARZADE, R. TAMOV, M. DAVTYAN, R. SAFAROV, S. IRAN TUNIEV, B. GABRIELYAN, E. SHAMCHIYEV, T. AGHILI, A. VAISMAN, A. GLYCHIAN, D. SULEIMANOV, M. EVERETT, J. (Coordinator) BELIK, V. GRIGORYAN, E. SULTANOV, E. FARVAR, M.T. JENDEREDJIAN, K. TAGIEVA, E. JAZEBIZADEH, K. KAZARYAN, H. KAVOUSI, K. TURKEY KAZARYAN, M. GEORGIA MAHFOUZI, M. ALTINTAS, M. KHASABYAN, M. ARABULI, A. MANSURI, J. ATAY, S KHOROZYAN, I. ARABULI, G. NAGHIZADEH, N BIRSEL, A. MANVELYAN, K. (Coordinator) BERUCHASHVILI, G. NAJAFI, A. CAN, E. MARKARYAN, N. BERUCHASHVILI, N. ZIYAEE, H. CIFTCI, N. MURADYAN, S. BUKHNIKASHVILI, A. RAHMANIYAN, M. DOMAC, A. RUKHKYAN, L. BUTKHUZI, L. GURKAN, B. SHASHIKYAN, S. CHEKURISHVILI, Z. IPEK, A. TOVMASYAN, S. DIDEBULIDZE, A. RUSSIA KALEM, S. VANYAN, A. DZNELADZE, M. BIRYUKOV, N. KUCUK, M. VARDANYAN, J. EGIASHVILI, D. BLAGOVIDOV, A. KURDOGLU, O. VOSKANOV, M. GELASHVILI, A. BRATKOV, V. KURT, B. ZIROYAN, A. GOGICHAISHVILI, L. BUKREEV, S. LISE, Y. (Coordinator) ZORANYAN, V. GOKHELASHVILI, R. CHILIKIN, V. URAS, A.