Pharmaceutical Sciences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cryptocarya of Thedirectorategeneral for Cryptocarya Development Cooperation , a Large, Pantropical Genus of Genus Pantropical Large, a , Cryptocarya Species
Taxonomy of Cryptocarya species of Brazil Taxonomy of Cryptocarya This revision of Brazilian species of Cryptocarya, a large, pantropical genus of species of Brazil Lauraceae, comes highly recommended. Lauraceae is an extensive family of trees that has remained poorly studied because large trees with small fl owers are often ignored by fi eld workers. In a time when so much botanical research is focused on relationships between taxa, it is refreshing to see such a detailed work on species delimitation in a previously inaccessible group. Everything one could want to know about neotropical Cryptocarya species is included: keys, descriptions, illustrations, use, etc. In short, this is a monograph in the classical sense. Pedro L.R. de Moraes The author has studied the species extensively in the fi eld and this fi eld knowledge adds much to the value of this taxonomic review and sets it apart from most revisions that often are largely based on studies of dried specimens. Here, detailed discussions of fi eld characters and photographs of fresh specimens are aptly integrated. In conclusion, this is an excellent contribution to our knowledge of Lauraceae and the author is to be congratulated. One could only wish for more publications on the same high level! December 2007 Dr H. Van der Werff Curator & Assistant Director of Research Missouri Botanical Garden, St. Louis, USA – Volume 3 (2007) – Volume Produced with the fi nancial support of the Directorate General for Development Cooperation Volume 3 (2007) 0885-07_ABC-TAXA3_Cover.indd 1 28-02-2008 14:40:08 -

Plant-Frugivore Interactions in a Heterogeneous Forest Landscape of South Africa
Plant-frugivore interactions in a heterogeneous forest landscape of South Africa Dissertation In partial fulfilment of the requirements for the award of a Doctorate Degree in Natural Sciences (Dr. rer. nat) The Faculty of Biology, Philipps-University of Marburg Lackson Chama, MSc Sinazongwe (Zambia) June 2012, Marburg From the Faculty of Biology, Philipps-University Marburg als Dissertation am angenommen. Dekan: Prof. Dr. Paul Galland Erstgutachterin: Prof. Dr. N. Farwig Zweitgutachter: Prof. Dr. R. Brandl Tag der Disputation: 25th June 2012 Dedicated to my son, Mishila, who’s first two years on earth I was hardly part of, due to my commitment towards this work. Contents CHAPTER 1: GENERAL INTRODUCTION ..................................................................................................................... 3 EFFECTS OF HUMAN ACTIVITIES ON FOREST BIODIVERSITY ........................................................................................................ 4 PLANT-FRUGIVORE INTERACTIONS IN CHANGING LANDSCAPES .................................................................................................. 5 THE ROLE OF FUNCTIONAL DIVERSITY IN FRUGIVORE COMMUNITIES ........................................................................................... 5 EFFECTS OF SEED INGESTION BY FRUGIVOROUS BIRDS ON GERMINATION SUCCESS ........................................................................ 6 AIMS OF THE THESIS ......................................................................................................................................................... -

Recruitment Dynamics of the Relict Palm, Jubaea Chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile
RESEARCH ARTICLE Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Intricate and Pervasive Effects of Invasive Herbivores and Nurse Shrubs in Central Chile Marina Fleury1,2*, Wara Marcelo2¤, Rodrigo A. Vásquez2, Luis Alberto González1, Ramiro O. Bustamante2 1 Facultad de Ciencias Forestales y de la Conservación de la Naturaleza, Universidad de Chile, Santiago, Chile, 2 Instituto de Ecología y Biodiversidad, Departamento de Ciencias Ecológicas, Universidad de Chile, Santiago, Chile ¤ Current address: Instituto de Conservación, Biodiversidad y Territorio, Facultad de Ciencias Forestales y Recursos Naturales, Universidad Austral de Chile, Valdivia, Chile * [email protected] OPEN ACCESS Citation: Fleury M, Marcelo W, Vásquez RA, Abstract González LA, Bustamante RO (2015) Recruitment Dynamics of the Relict Palm, Jubaea chilensis: Shrubs can have a net positive effect on the recruitment of other species, especially relict Intricate and Pervasive Effects of Invasive Herbivores species in dry-stressful conditions. We tested the effects of nurse shrubs and herbivory and Nurse Shrubs in Central Chile. PLoS ONE 10(7): defoliation on performance (survival and growth) of nursery-grown seedlings of the largest e0133559. doi:10.1371/journal.pone.0133559 living palm, the relict wine palm Jubaea chilensis. During an 18-month period, a total of Editor: Jin-Song Zhang, Institute of Genetics and more than 300 seedlings were exposed to of four possible scenarios produced by indepen- Developmental Biology, Chinese Academy of Sciences, CHINA dently weakening the effects of nurse shrubs and browsers. The experiment followed a two- way fully factorial design. We found consistent differences in survival between protected Received: March 2, 2015 and unprotected seedlings (27.5% and 0.7%, respectively), and herbivory had a dramatic Accepted: June 28, 2015 and overwhelmingly negative effect on seedling survival. -

Revista Chilena De Entomología
Rev. Chilena Ent. 2000, 26: 85 - 88 THE EFFECT OF HERBIVORY RATES ON THE PRODUCTION OF LEAVES AND FLOWERS BY THREE SPECIES OF THE MATORRAL OF CENTRAL CHILE' Manuel A. Olivera^ And Blas I. Lavandero^ ABSTRACT The relationship between herbivory damage and the production of new leaves and flowers in Quillaja sapo- naria Mol, Escallonia pulverulenta (R. et P.) Pers. and Cryptocarya alba (Mol.) Looser was studied. No relation was found production of new leaves and flowers by the three species and herbivory damage. A múltiple regression was significant in E. pulverulenta: the number of leaves with galls damage explained 99 % of the variability in the number of flowers. In spite of finding differences between species in the ratio between the number of new leaves and the number of flowers and in the proportion of damaged oíd leaves, these did not lead to any type of correlation. Key words.- herbivory, vegetative growth, flowering, central Chilean Matorral, Quillaja saponaria. E. pulverulenta, Cryptocarya alba. RESUMEN Se estudió la posible relación entre daño por herbivoría y producción de hojas nuevas y flores en Quillaja saponaria Mol, E. pulverulenta (R. et P) Pers. y Cryptocarya alba (Mol.) Looser. No se encontró relación entre estas variables en ninguna de las tres especies. Una regresión múltiple resultó significativa en E. pulverulenta: el número de hojas dañadas con agallas predijo el 99% de la variabilidad en el número de flores. A pesar de que se encontró diferencias entre las especies en la razón hojas nuevas/flores, éstas no fueron significativas. Palabras claves.- herbivoría, crecimiento vegetativo, floración, matorral de Chile central, Quillaja saponaria, E. -

68.K.N. RAVINDRA-Study of Antioxidant Activity And
IAJPS 2019, 06 (10), 12735-12744 Ravindra K N et al ISSN 2349-7750 CODEN [USA]: IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICAL SCIENCES Available online at: http://www.iajps.com Research Article STUDY OF ANTIOXIDANT ACTIVITY AND ESSENTIAL OILS COMPOSITION FROM THE FLOWERS AND FRUITS OF CRYPTOCARYA STOCKSII MEISN Ravindra K N*, Sharanappa P 1Department of Bioscience, P.G.Centre, University of Mysore, Hemagangothri, Hassan, India-573226. Article Received: August 2019 Accepted: September 2019 Published: October 2019 Abstract: The aim of this study was to investigate the flowers and fruits essential oil composition and antioxidant activity of Cryptocarya stocksii, an endemic and vulnerable tree grows in the Western Ghats of India. The essential oils were obtained by hydrodistillation using a Clevenger apparatus and analyzed by GC/MS. GC-MS analysis of essential oil of flower and fruit revealed the presence of individual 24 different compounds corresponding to 99.87% and 99.99% of the total oil respectively. The flower and fruit oil were dominated by α-Pinene (39.42% and 8.84%), β-Pinene (17.96% and 4.87%), 3-Carene (29.52% and 1.92%) and Longifolene (6.53% and 19.12%) respectively. Antioxidant activities were determined by four different test systems, namely, DPPH, FRAP, H2O2, and anti-lipid peroxidation assay and result found to be dose dependent manner. This work represents the first study of the chemical composition and anti oxidant of essential oils from flowers and fruits of C. stocksii. Keywords: Cryptocarya stocksii, Lauraceae, essential oils, antioxidant activity, GC-MS. Corresponding author: *K N Ravindra, QR code Department of Bioscience, P.G.Centre, University of Mysore, Hemagangothri, Hassan, India-573226. -

Taxonomy and Biology of a New Oecophoridae (Lepidoptera) from Central Chile
Revista Chilena de Historia Natural 74:533-538, 2001 Taxonomy and biology of a new Oecophoridae (Lepidoptera) from central Chile Taxonomfa y biologfa de un nuevo Oecophoridae (Lepidoptera) de Chile central T. HEATH-OGDEN 1 & LUIS E. PARRA Departamento de Zoología, Facultad de Ciencias Naturales y Oceanognificas, Universidad de Concepcion, Casilla 160-C, Concepcion, Chile, e-mail: [email protected] 1Current address: Department of Zoology, 574 Widtsoe Building, Brigham Young University, Provo, Utah 84602, U.S.A., e-mail: [email protected] ABSTRACT The adult, larva, and pupa of Afderajimenae Ogden & Parra sp. nov. are described and illustrated. Larvae live in leaf litter throughout all instars and are generalists feeding upon the fallen leaves of a number of different plant species of sclerophyllous forests. Comments on morphological details and bionomics ofthis species are given. This is the second species of Afdera know for Chile. Key words: Afdera jimenae, biology, leaf litter, Oecophoridae, sclerophyllous forest, taxonomy. RESUMEN Se describe e ilustra el adulto, larva y pupa de Afdera jimenae Ogden & Parra sp. nov. Los diferentes estados larvarios se encuentran en Ia hojarasca del bosque esclerofilo de Ia Península de Hualpen, alimentandose de hojas en descomposicion de diferentes especies vegetales. Se entregan comentarios sobre detalles morfologicos y bionomicos de Ia especie. Esta es Ia segunda especie de Afdera descrita para Chile. Palabras clave: Afderajimenae, biologfa, bosque esclerofilo, hojarasca, Oecophoridae, taxonomfa. INTRODUCTION cies yet to be discovered. This paper increases the knowledge of this interesting group, giving a The Chilean Oecophoridae are different from most detailed description of one species, including other gondwanan oecophorids because they lack many important biological and ecological consid- ocelli (Clark 1978), there are only a few species erations. -

Recommended Yard and Open Space Tree Species List
Recommended Yard and Open Space Tree Species List - With Notations San Francisco Urban Forestry Council Updated February 2016 San Francisco has a unique climate and conditions for trees, and our open spaces (including parks, private lawns, and open spaces under federal jurisdiction, such as The Presidio) present an opportunity to plant a wide variety of trees that are not yet common here. Many of these trees are not well suited as street trees (either because they cannot tolerate compacted soils, or have sidewalk- buckling roots). The following list of trees are considered both well-adapted to San Francisco’s climate and conditions, and also under-represented in our open spaces. They present an opportunity for experimentation and improving San Francisco’s urban forestry diversity. Size Evergreen/ Species Notes Deciduous Small - Evergreen Leptospermum scoparium Broad, low crown; pink flowers; often multi - ‘Helene Strybing’ trunked Less than (New Zealand tea tree) 20’ tall at maturity Small - Evergreen Acer laevigatum Broad crown; semi -weeping (smooth maple or Nepal Less than maple) 20’ tall at maturity Alectryon excelsus Broad crown; small red fruits. Moderate growth (titoki) speed Carpodetus serratus Endemic to New Zealand (marbleleaf) Cussonia spicata Dramatic foliage texture Elaeocarpus decipiens Upright habit; tiny white flowers; blue -black fruits (Japanese blueberry tree) Melaleuca ericifolia Broad, dense crown; covered in white flowers in (swamp paperbark) early summer; attractive whitish peeling bark Meryta sinclairii Round headed; bold foliage texture (puka) Sophora microphylla& Rounded crown; yellow flowers S.tetraptera. (kowhai) Pittosporum erioloma (Lord Upright, irregular pagoda shape Howe Island Pittosporum) Vitex lucens Broad crown; reddish flowers (New Zealand chaste tree) Small - Deciduous Acer griseum Broad crown; reddish peeling bark; good fall (paperbark maple) color. -

Palm Forests (Jubaea Chilensis (Mol.) Baillon) in the Region of Valparaíso – Chile
The Rufford Small Grants Foundation Final Report Ecological parameters related to the regeneration of Chilean palm forests (Jubaea chilensis (Mol.) Baillon) in the Region of Valparaíso – Chile Name of grantee: Francisco Aguirre Saavedra Associated scientists: Gonzalo Ibáñez Villaseca Susana Bogdanic Hernández Cristian Ray Bobadilla Plan Nacional de Conservación de la Palma Chilena Operational context Antecedents Jubaea chilensis (Mol.) Baillon, is the world’s second southernmost palm, endemic from Chile and it is the only species of its genus. In days gone by, the Chilean palm covered 600 km of the country forming several plant units. Varied anthropogenic interventions have caused fragmentation of the palm population, decrease of seed bank and decline of juvenile individuals. Nowadays, few places in Chile can be considered as palm forests, due to the shortage of its population, which do not exceed 65.000 specimens. Three of the four Chilean palm forests can be found in the region of Valparaíso, Image 1. The harvest of fruits for sale is a common practice in all the palm forests. In the including the largest one with age heterogeneity — it was picture, the nails are inserted in the stalk to here where the project was carried out. According to the climb the apical part. Chilean legislation and the IUCN classification (2008), J. chilensis is in vulnerable conservation status; it is protected of any anthropogenic action that could directly interfere in the development of the species or in the place where they grow. Due to the continuous decrease on the number of individuals, in 2006 the National Plan for the Conservation and Recovery of the Chilean palm was created. -

Medicinal Plant Conservation
MEDICINAL Medicinal Plant PLANT SPECIALIST Conservation GROUP Volume 15 Newsletter of the Medicinal Plant Specialist Group of the IUCN Species Survival Commission Chaired by Danna J. Leaman Chair’s note .......................................................................................................................................... 2 Taxon file Conservation of the Palo Santo tree, Bulnesia sarmientoi Lorentz ex Griseb, in the South America Chaco Region - Tomás Waller, Mariano Barros, Juan Draque & Patricio Micucci ............................. 4 Manejo Integral de poblaciones silvestres y cultivo agroecológico de Hombre grande (Quassia amara) en el Caribe de Costa Rica, América Central - Rafael Ángel Ocampo Sánchez ....................... 9 Regional file Chilean medicinal plants - Gloria Montenegro & Sharon Rodríguez ................................................. 15 Focus on Medicinal Plants in Madagascar - Julie Le Bigot ................................................................. 25 Medicinal Plants utilisation and conservation in the Small Island States of the SW Indian Ocean with particular emphasis on Mauritius - Ameenah Gurib-Fakim ............................................................... 29 Conservation assessment and management planning of medicinal plants in Tanzania - R.L. Mahunnah, S. Augustino, J.N. Otieno & J. Elia...................................................................................................... 35 Community based conservation of ethno-medicinal plants by tribal people of Orissa state, -

Edible Nuts. Non-Wood Forest Products
iii <J)z o '"o ~ NON-WOODNO\ -WOOD FORESTFOREST PRODUCTSPRODUCTS o 55 Edible nuts Food and Agriculture Organization of the United Nations NON-WOOD0 \ -WOOD FOREST FOREST PRODUCTS PRODUCTS 55 EdibleEdible nuts by G.E. Wickens FOOD AND AGRICULTUREAGRICULTURE ORGANIZATION OF THE UNITEDUNITED NATIONSNATIONS Rome,Rome, 19951995 The opinions expressed in this document are those of the authors and do not necessarily reflectreflect opinionsopinions onon thethe partpart ofof FAO.FAO. The designations employed and the presentation of material in this publication do notnot implyimplythe the expressionexpression ofof any anyopinion opinion whatsoever whatsoever onon thethe part of thethe FoodFood andand AgricultureAgriculture OrganizationOrganization of thethe UnitedUnited Nations concerning the legal status of any country,country, territory,territory, citycity oror area or ofof itsits authorities, authorities, orconcerningor concerning the the delimitation delimitation ofof its its frontiers frontiers or boundaries.boundaries. M-37 ISBNISBN 92-5-103748-5 All rights reserved. No part of this publication may be reproduced,reproduced , stored in a retrieval systemsystem,, or transmitted inin any formform oror byby anyany means, means ,electronic, electronic, mechanicalmechanical,, photocopying oror otherwiseotherwise,, without the prior permissionpermission ofof thethe copyright owner. Applications forfor such permission,permission, withwith a statementstatement of thethe purpose and extent of the reproduction,reproduction, should be addressed to the -

Immature Stages, Phenology, Distribution and Host Plants of the Andean Moon Moth Cercophana Frauenfeldii Felder, 1862 (Lepidoptera: Saturniidae)
Revista Brasileira de Entomologia 65(2):e20190017, 2021 Immature stages, phenology, distribution and host plants of the Andean Moon Moth Cercophana frauenfeldii Felder, 1862 (Lepidoptera: Saturniidae) Joaquín E. Sepúlveda1,2 , Enrique A. Mundaca2* , Diego Muñoz-Concha2 , Luis E. Parra3 , Héctor A. Vargas4 1Instituto Agroecosistemas, Curicó, Chile. 2Universidad Católica del Maule, Facultad de Ciencias Agrarias y Forestales, Escuela de Agronomía, Curicó, Chile. 3Universidad de Concepción, Facultad de Ciencias Naturales y Oceanográficas, Departamento de Zoología, Concepción, Chile. 4Universidad de Tarapacá, Facultad de Ciencias Agronómicas, Departamento de Recursos Ambientales, Arica, Chile. ARTICLE INFO ABSTRACT Article history: Cercophana frauenfeldii Felder (Lepidoptera: Saturniidae), also known as the “Andean Moon Moth”, is a Neotropical Received 06 November 2019 species native to continental Chile whose larvae feed on species of the families Gomortegaceae, Laureaceae and Accepted 09 May 2021 Winteraceae. We describe and document C. frauenfeldii immature stages, namely, egg, its four larval instars, and Available online 28 May 2021 chaetotaxy of the last instar, pupa and cocoon for the first time. In terms of its phenology, we extend its larval Associate Editor: Thamara Zacca activity, originally described to occur between November and mid-December, to June until the end of January. We report the adult flight period depends on the species’ distributional range following two well-differentiated patterns: February to mid-April in Central-North Chile and April to June in Central-South Chile. Furthermore, we Keywords: provide a unified view of its current distributional range and host plants (including the endangered tree Gomortega Biology keule) through bibliographic data, field observations and laboratory rearing. Finally, we discuss aspects of the Camouflage species’ conservation as part of the unique ecosystems found in the temperate forests of southern South-America. -
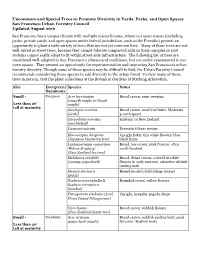
Uncommon and Special Trees to Promote Diversity in Yards, Parks, and Open Spaces San Francisco Urban Forestry Council Updated A
Uncommon and Special Trees to Promote Diversity in Yards, Parks, and Open Spaces San Francisco Urban Forestry Council Updated August 2016 San Francisco has a unique climate with multiple microclimates, where our open spaces (including parks, private yards, and open spaces under federal jurisdiction, such as the Presidio) present an opportunity to plant a wide variety of trees that are not yet common here. Many of these trees are not well suited as street trees, because they cannot tolerate compacted soils or their canopies or root systems cannot easily adapt to fit within street-side infrastructure. The following list of trees are considered well-adapted to San Francisco’s climate and conditions, but are under-represented in our open spaces. They present an opportunity for experimentation and improving San Francisco’s urban forestry diversity. Though some of these species may be difficult to find, the Urban Forestry Council recommends considering these species to add diversity to the urban forest. To view many of these trees in person, visit the plant collections at the Botanical Gardens at Strybing Arboretum. Size Evergreen/ Species Notes Deciduous Small - Evergreen Acer laevigatum Broad crown; semi -weeping (smooth maple or Nepal Less than 20’ maple) tall at maturity Alectryon excelsus Broad crown; small red fruits. Moderate (titoki) growth speed Carpodetus serratus Endemic to New Zealand (marbleleaf) Cussonia spicata Dramatic foliage texture Elaeocarpus decipiens Upright habit; tiny white flowers; blue - (Japanese blueberry tree) black fruits Leptospermum scoparium Broad, low crown; pink flowers; often ‘Helene Strybing’ multi-trunked (New Zealand tea tree) Melaleuca ericifolia Broad, dense crown; covered in white (swamp paperbark) flowers in early summer; attractive whitish peeling bark Meryta sinclairii Round headed; bold foliage texture (puka) Sophora microphylla & Rounded crown; yellow flowers Sophora tetraptera.