Gut Microbiome an Intermediary to Neurotoxicity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Impact of Pesticide Use on Health in Developing Countries
Impact of pesticide use on health in developing countries Proceedings of a symposium held in Ottawa, Canada, 1 7-20 September 1990 IDRC CRDI International Development Research Centre Centre de recherches pour le devetoppement international 1 March 1993 Dear Reader/Librarian, IDRC is a public corporation created by the Canadian parliament in 1970 to help developing countries find viable solutions to their problems through research. At the 1992 Earth Summit, IDRC's mandate was broadened to emphasize sustainable development issues. As part of IDRC's strengthened commitment to global action and harüony, we are pleased to send you a complimentary copy of our most recent publication: The impact of pesticide use on health in developing countries (March 1993, 352 pages, 0-88936-560-1, $17.95). The first part of this book presents a brief survey of the global situation and the results of twelve epidemiological studies carried out by researchers from Africa, Latin America, Asia and the Middle East. These focus on poisonings resulting from organophosphates, herbicides, and pyrethroids. The second part illustrates the role of the process of development, production, spraying techniques and legislation in protecting the health of workers. A discussion of the benefits and modalities of access to pertinent information for the prevention of pesticide poisonings is provided in the third section. Finally, in the fourth section, consideration is given to the advantages and disadvantages of certain alternatives to the use of synthetic pesticides in agriculture and public health, such as botanical pesticides and integrated pest management strategies. We hope this book is a valuable addition to your collection. -
![[별표 1] 유독물(제2조 관련) 1. 과산화 나트륨 [Sodium Peroxide] 및 이를 함유한 제제](https://docslib.b-cdn.net/cover/4854/1-2-1-sodium-peroxide-1024854.webp)
[별표 1] 유독물(제2조 관련) 1. 과산화 나트륨 [Sodium Peroxide] 및 이를 함유한 제제
[별표 1] 유독물(제2조 관련) 1. 과산화 나트륨 [Sodium peroxide] 및 이를 함유한 제제. 다만, 이를 5%이하 함유한 것은 제외한다. 2. 과산화 수소 [Hydrogen peroxide] 및 이를 함유한 제제. 다만, 이를 6%이하 함유한 것은 제외한다. 3. 과산화 요소 [Urea peroxide] 및 이를 함유한 제제. 다만, 이를 17%이하 함유한 것은 제외한다. 4. 나트륨 [Sodium] 5. β-나프톨 [β-Naphthol] 및 이를 함유한 제제. 다만, 이를 1%이하 함유한 것은 제외한다. 6. 1-나프틸 메틸카르밤산 [1-Naphthyl methylcarbamate : Carbaryl, Sevin, Arylam] 및 이를 함유한 제제. 다만, 이를 5%이하 함유한 것은 제외한다. 7. α-나프틸 티오우레아 [α-Naphthyl thiourea : ANTU, Bantu, Krysid] 및 이를 함유한 제제 8. 납 화합물 [Lead compound]. 다만, 시산화 심납(Lead tetroxide), 황산 납(Lead sulfate), 염기성 탄산납(Basic lead carbonate)은 제외한다. 9. 니켈 카르보닐 [Nickel carbonyl] 및 이를 함유한 제제 10. 니코틴 [Nicotine]과 그 염류 및 그중 하나를 함유한 제제 11. 니트로벤젠 [Nitrobenzene] 12. 1-(4-니트로페닐)-3-(3-피라딜메틸)우레아 [1-(4-Nitrophenyl)-3-(3-pyridylmethyl)urea : Priminil, Vacor] 및 이를 함유한 제제 13. 2,4-디니트로-6-메틸프로필페닐디메틸 아크릴산 [2,4-Dinitro-6-methyl propylphenyldimethyl acrylate : Binapacryl, Morocide] 및 이를 함유한 제제 14. 4,6-디니트로-o-크레솔 [4,6-Dinitro-o-cresol : DNOC, Antinonnin, Sinox]과 그 염류 및 그중 하나를 함유한 제제 15. 3-(디메톡시포스핀일옥시)-N-메틸이소크로톤아미드 [3-(Dimethoxyphosphinyloxy)-N-methylisocrotonamide : Azodrin, Monocrotophos, Apadrin] 및 이를 함유한 제제 16. O,O-디메틸 O-p-니트로페닐 티오인산 [O,O-Dimethyl O-p-nitrophenyl thiophosphate : Methyl parathion, Metaphos, Metacide] 및 이를 함유한 제제 17. 디메틸 (E)-2-디메틸카르바모일-1-메틸비닐 인산 [Dimethyl (E)-2-dimethylcarbamoyl-1-methylvinyl phosphate : Ektafos, Dicrotophos, Bidrin, Carbicron] 및 이를 함유한 제제 18. -

TM-C Annexes.Pdf
DESTRUCTION AND DECONTAMINATION TECHNOLOGIES FRO PCBs AND OTHER POPs WASTES UNDER THE BASEL CONVENTION A Training Manual for Hazardous Waste Project Managers Volume C - Annexes Secretariat of the Basel Convention Autoclaving Name of Process: Autoclaving Status: Autoclaving is a technology that has been around for many years now and is Applicable POPs wastes: well proven. In general for PCBs, only the oil and transformer components such PCB’s and not for other POPs as ceramics, cardboard and wood are incinerated. After decontamination, the various metals such as copper, steel and aluminium are sent to the metals recycling industry. In fact, the autoclaving cannot be compared with the other technologies dealt with, as it does not treat the PCB itself, but its objective is the clean-up and re- use of the with PCB contaminated materials, which is a completely different purpose as the other technologies. One has to consider that 98% of the contaminated oil is not going into the autoclaving but directly to the incinerator! Technology description: Autoclaving is a solvent decontamination process that extracts PCBs from contaminated material. The process is most often used in projects in conjunction with high temperature incineration. For capacitors the process involves shredding and placing all the material into the autoclaving chamber and by vacuum extraction with solvent remove the PCB. The condensers solid materials, after sawing and decontamination, are totally treated in the incineration plant through the solid burden way, and only the ceramics can then be landfilled. The extracted oil and PCBs is sent for HTI incineration, Alkali metal reduction or other alternative technologies. -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -

China Releases New Maximum Residue Limits for Pesticides In
GB 2763-2016 THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Voluntary - Public Date: 3/31/2017 GAIN Report Number: CH17016 China - Peoples Republic of Post: Beijing China Releases New Maximum Residue Limits for Pesticides in Food Report Categories: FAIRS Subject Report Approved By: Lisa Anderson Prepared By: FAS Staff Report Highlights: On December 18, 2016, the Chinese National Health and Family Planning Commission, Ministry of Agriculture, China Food and Drug Administration released the National Food Safety Standard - Maximum Residue Limits for Pesticides in Foods (GB 2763-2016). The standard will replace the current MRL Standard (GB 2763-2014) and will be implemented on June 18, 2017. This report provides an unofficial translation of the standard. Editors’ Note: The asterisk appearing in the MRL column means that the limit is a temporary MRL. A temporary MRL is usually set under the following four conditions: 1. The dietary risk assessment data is incomplete; 2. The Acceptable Daily Intake (ADI) is temporary (ADI is used as the basis for MRL setting); 3. There is no surveillance or analysis method for the MRL that complies with the standard requirements; 4. In emergency situations, the pesticide is approved to be used on un-registered crops. I GB 2763-2016 General Information: BEGIN TRANSLATION ICS 65.100 G 25 GB National Standard of the People’s Republic of China GB 2763—2016 Replacing GB 2763 - 2014 National food safety standard Maximum Residue Limits for Pesticides in Food General Information: National Health and Family Planning Commission Issued by: Ministry of Agriculture China Food and Drug Administration Issued on: 2016-12-18 Implementation:2017-06-18 II GB 2763-2016 Table of Content Preface ............................................................................................................................................................... -

The Insecticides Act, 1968 (Act No.46 of 1968)
The Insecticides Act, 1968 (Act No.46 of 1968) An Act to regulate the import, manufactures, sale, transport, distribution and use of insecticides with a view to prevent risk to human beings or animals and for matters connected therewith. [2 nd September 1968] Be it enacted by Parliament in the Nineteenth Year of the Republic of India as follows: 1. Short title, extent and commencement. * a. This Act may be called the Insecticides Act, 1968. b. It extends to the whole of India. c. It shall come into force on such date as the Central Government may, by notification in the official Gazette, appoint and different dates may be appointed for different States and for different provisions of Act. 2. Application of other laws not barred * The provisions of this Act shall be in addition to, and not in derogation of, any other law for the time being in force. 3. Definitions- In this Act, unless the context otherwise requires- a. "animals" means animals useful to human beings and includes fish and fowl, and such kinds of wild life as the Central Government may, by notification in the official Gazette, specify, being kinds which in its opinion, it is desirable to protect or preserve; b. "Board" means the Central Insecticides Board constituted under Sec.4; c. "Central Insecticides Laboratory" means the Central Insecticides Laboratory established, or as the case may be, the institution specified under Sec.16; d. "Import" means bringing into any place within the territories to which this Act extends from a place outside those territories; e. "Insecticide" means- i. -
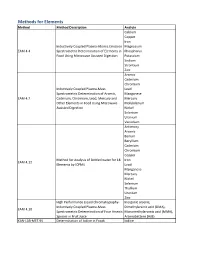
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

Supporting Information Manuscript Title Glutathione-S-Transferase
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2017 Supporting Information Manuscript title Glutathione-S-Transferase catalyzed reaction of glutathione for electrochemical biosensing of Temephos, Fenobucarb and Dimethoate Authors Himadri Boraha, Rekha Rani Duttaa, Sudarsan Gogoia, Tapas Medhib and Panchanan Puzaria* aDepartment of Chemical Sciences, Tezpur University, Tezpur, Assam, India-784028 b Department of Molecular Biology and Biotechnology, Tezpur University, Tezpur, Assam, India-784028 Km and Vmax for GSH-CDNB-GST (without inhibitor): 2.5 2 1.5 1 0.5 0 0 5 10 15 20 25 Km = slope/intercept= 0.071/0.876 = 0.08 Vmax = Km/slope = 0.08/0.071 =1.14 Ki calculation for Fenobucarb: Km 60 ppb= 0.219/0.875 = 0.2502 Vmax = 0.2502/0.219 = 1.14 Km 50 ppb= 0.135/0.872 = 0.1548 Vmax = 0.1548/0.135= 1.14 Km 40ppb = 0.083/0.875= 0.0948 Vmax = 0.0948/0.083= 1.14 6 5 4 3 2 1 0 0 5 10 15 20 25 Km (inhibitor) / Km (without inhibitor) vs [I] = Slope = 1/Ki Km (without inhibitor) = 0.08 K60 ppb/Km= 0.2502/0.08 = 3.12 K50 ppb/Km = 0.1548/0.08= 1.93 K40ppb/Km = 0.0948/ 0.08= 1.18 Concentration of fenobucarb Km value for each concentration 60 ppb 3.12 50 ppb 1.93 40 ppb 1.18 3.5 3 2.5 2 1.5 1 0.5 0 0 10 20 30 40 50 60 70 Slope = 1/Ki = 0.097 Therefore, Ki = 1/0.097 = 10.30 Ki calculation for Temephos: Km 60 ppb= 0.104/ 1.278 = 0.0813 Vmax= 0.0813/0.104= 0.7817 Km 50ppb = 0.090/1.094 = 0.0822 Vmax = 0.0822/0.090 = 0.9133 Km 40 ppb= 0.077/0.948 = 0.0812 Vmax = 0.0812/0.077 = 1.054 3.5 3 2.5 -

Agro-Chemical Technical with Brand Name, Application
AGRO-CHEMICAL TECHNICAL WITH BRAND NAME, APPLICATION, DOSE AND MODE OF ACTION INSECTICIDES / ACARICIDES / MITICIDES Technical Brand Name Application Dose / Acre Mode of Action ✓ Red mite of strawberry, cotton, cucumber, potato, 50-100 1. Abamectin 1.8% EC Vertimec (Syngenta), Tagmec 1.9 EC (Tropical), ABC 1.85% EC (KR) soybean, tomato and sweet melon ml/acre, 5-10 Chloride channel activators group. Acaricide ✓ Leaf miners of sugarbeet ml/pump Starthene (Swal), Orthene (Arysta), Missile (Devidayal), Megastar (MIL), ✓ It is particularly effective on severe infestations of Lancer (UPL), Oval (PI Ind.), Rasayan Phate (KR), Acefex (Excel), sucking and chewing insects of tobacco, sugarcane, Kingmax (Vimax), Asataf (TATA), Accent 787 (Sumil), Miltaf (IIL), Bheem cotton, chilies, vegetables, fruits and cereals. 300-400 (Kilpest), Tagace (Tropical), Lucid (Cheminova), Lion (SuperCSL), Sritaf ✓ It has low toxicity to mammals and does not harm gm/acre A versatile organophosphate group insecticide 2. Acephate 75% SP (Crystal), Ortain (Coromandel), Hilphate (HIL), Ample (Advance), Rythane beneficial insects. It is easy to use, being soluble in 20-25 with both contact and systemic action. (Ramcides), Corohamp (CAPL), Topsis (Atul), Molphate (GP), Top‘O’Top water. gm/pump (CGI), King Phate (KCS), Acesul (Sulphur Mills), Vega (PCCPL), Pace ✓ Green & Brown Leafhoppers, Brown Plant Hoppers (Nagarjuna), Topsis (Atul), Tremor (BioStadt), Ace (Canary), Willace ✓ ,L,L 5M58L TYF T0T0LIF H[JL R];LIF HLJFTGF lGI\+6 (Willowood), Chettak (GSP), Archa (Amber), Bhoochal 75 (AOL) DF8[P ✓ It targets Stem borer, leaf folder & BPH on Paddy. ✓ It has strong systemic molecule and is highly soluble and longer Belongs to the Organophosphates insecticide 3. -
EVALUATION of PESTICIDE RESIDUES in SPICES First Draft Prepared by Prof
Spices 1779 EVALUATION OF PESTICIDE RESIDUES IN SPICES First draft prepared by Prof. Dr. Arpad Ambrus, Hungarian Food Safety Office, Budapest, Hungary EXPLANATION The 2004 JMPR estimated maximum residue levels of pesticides in or on spices on the basis of monitoring results, and recommended that the maximum residue levels should be estimated to cover 95% of the residue population at 95% confidence level. The CCPR at its Thirty-sixth Session proposed that commodity group A028 be subdivided into sub-groups based on the parts of plants from which they are obtained – seeds, fruits or berries, roots or rhizomes, bark, buds, arils and flower stigmas – and that MRLs for pesticides that had been evaluated within the Codex system should be set for these sub-groups rather than for each of the pesticide–spice combinations15. The present Meeting followed the same principles which were applied by the 2004 JMPR and endorsed by the CCPR. Registered use of some pesticides on peppers and monitoring data on spices were submitted by Thailand16. The European Spice Association presented the results of a survey on the effect of dehydration on some fresh spices. This information was evaluated by the present Meeting. METHODS OF RESIDUE ANALYSIS The on-line multi residue methods applied for the determination of pesticide residues is based on extraction with a mixture of acetone, dichloromethane and sodium chloride water solution. The concentrated extract is cleaned up on silica gel column and detection with GC-ECD (Steinwandter, 1985). The carbendazim residues were extracted with acetone + sulphuric acid and partitioned with dichloromethane (Wong), or determined with the QUECHER method (Anastassiades et al., 2003).