Abundant Trace Fossil Piscichnus 15
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sharks in Crisis: a Call to Action for the Mediterranean
REPORT 2019 SHARKS IN CRISIS: A CALL TO ACTION FOR THE MEDITERRANEAN WWF Sharks in the Mediterranean 2019 | 1 fp SECTION 1 ACKNOWLEDGEMENTS Written and edited by WWF Mediterranean Marine Initiative / Evan Jeffries (www.swim2birds.co.uk), based on data contained in: Bartolí, A., Polti, S., Niedermüller, S.K. & García, R. 2018. Sharks in the Mediterranean: A review of the literature on the current state of scientific knowledge, conservation measures and management policies and instruments. Design by Catherine Perry (www.swim2birds.co.uk) Front cover photo: Blue shark (Prionace glauca) © Joost van Uffelen / WWF References and sources are available online at www.wwfmmi.org Published in July 2019 by WWF – World Wide Fund For Nature Any reproduction in full or in part must mention the title and credit the WWF Mediterranean Marine Initiative as the copyright owner. © Text 2019 WWF. All rights reserved. Our thanks go to the following people for their invaluable comments and contributions to this report: Fabrizio Serena, Monica Barone, Adi Barash (M.E.C.O.), Ioannis Giovos (iSea), Pamela Mason (SharkLab Malta), Ali Hood (Sharktrust), Matthieu Lapinksi (AILERONS association), Sandrine Polti, Alex Bartoli, Raul Garcia, Alessandro Buzzi, Giulia Prato, Jose Luis Garcia Varas, Ayse Oruc, Danijel Kanski, Antigoni Foutsi, Théa Jacob, Sofiane Mahjoub, Sarah Fagnani, Heike Zidowitz, Philipp Kanstinger, Andy Cornish and Marco Costantini. Special acknowledgements go to WWF-Spain for funding this report. KEY CONTACTS Giuseppe Di Carlo Director WWF Mediterranean Marine Initiative Email: [email protected] Simone Niedermueller Mediterranean Shark expert Email: [email protected] Stefania Campogianni Communications manager WWF Mediterranean Marine Initiative Email: [email protected] WWF is one of the world’s largest and most respected independent conservation organizations, with more than 5 million supporters and a global network active in over 100 countries. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Batoid Abundances, Spatial Distribution, and Life History Traits
animals Article Batoid Abundances, Spatial Distribution, and Life History Traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a Knowledge Gap through Three Decades of Survey Michele Luca Geraci 1,2 , Sergio Ragonese 2,*, Danilo Scannella 2, Fabio Falsone 2, Vita Gancitano 2 , Jurgen Mifsud 3, Miriam Gambin 3, Alicia Said 3 and Sergio Vitale 2 1 Geological and Environmental Sciences (BiGeA)–Marine Biology and Fisheries Laboratory, Department of Biological, University of Bologna, Viale Adriatico 1/n, 61032 Fano, PU, Italy; [email protected] 2 Institute for Marine Biological Resources and Biotechnology (IRBIM), National Research Council–CNR, Via Luigi Vaccara, 61, 91026 Mazara del Vallo, TP, Italy; [email protected] (D.S.); [email protected] (F.F.); [email protected] (V.G.); [email protected] (S.V.) 3 Department of Fisheries and Aquaculture, Ministry for Agriculture, Fisheries and Animal Rights (MAFA), Ghammieri Government Farm, Triq l-Ingiered, Malta; [email protected] (J.M.); [email protected] (M.G.); [email protected] (A.S.) * Correspondence: [email protected] Simple Summary: Batoid species are cartilaginous fish commonly known as rays, but they also Citation: Geraci, M.L.; Ragonese, S.; include stingrays, electric rays, guitarfish, skates, and sawfish. These species are very sensitive Scannella, D.; Falsone, F.; Gancitano, to fishing, mainly because of their slow growth rate and late maturity; therefore, they need to be V.; Mifsud, J.; Gambin, M.; Said, A.; adequately managed. Regrettably, information on life history traits (e.g., length at first maturity, Vitale, S. Batoid Abundances, Spatial sex ratio, and growth) and abundance are still scarce, particularly in the Mediterranean Sea. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

Protection of Sharks and Rays in the Israeli Mediterranean
Plan of Action for Protection of Sharks and Rays in the Israeli Mediterranean 2016 II Written by: Asaf Ariel, Adi Barash With comments from: Aviad Scheinin, Oren Sonin, Eric Diamant, Dor Adalist, Danny Golani, Danny Chernov, Menachem Goren, Eran Brokovitch, Tomer Kochen and Ruth Yahel Translation: Jennifer Levin Graphic Design: Yael Jicchaki-Golan Photography: Uri Ferro, Aviram Waldman, Aviad Scheinin, Adi Barash, Haggai Netiv, Shai Milat, Guy Hadash, Hod Ben Hurin, Charles Roffey, Brian Gratwicke Cover and back jacket photography: Uri Ferro Recommended citation: Ariel, A. and Barash, A. (2015). Action Plan for Protection of Sharks and Rays in the Israeli Mediterranean. EcoOcean Association. III Photography: Aviram Valdman, www.thetower.org/article/photos-worlds-beneath-the-sacred-waters,'Tower Magazine' IV Table of Contents Executive Summary .................................................................................1 1. Introduction.......................................................................................3 1.1 The Objective of the Proposed Action Plan ....................................3 1.2 About the Model for the Action Plan .............................................3 2. Background .......................................................................................5 2.1 Sharks and rays and their ecological importance ......................5 2.2 Sharks and rays in the Mediterranean and in the coastal waters of Israel ............................................................................6 2.3 Factors that -

Pocket Identification Guide of Main Vulnerable Species Incidentally Caught in Tunisian Fisheries
POCKET IDENTIFICATION GUIDE OF MAIN VULNERABLE SPECIES INCIDENTALLY CAUGHT IN TUNISIAN FISHERIES Simplified guide adapted for Tunisia (GSA 12,13 & 14) from the Identification guide of vulnerable species incidentally caught in Mediterranean fisheries Logos en anglais, avec versions courtes des logos ONU Environnement et PAM La version longue des logos ONU Environnement et PAM doit être utilisée dans les documents ou juridiques. La version courte des logos est destine tous les produits de communication tourns vers le public. IN COLLABORATION WITH FUNDED BY 8 List of seabirds – toolkit for observers: Scientific name Spanish French name Arabic name SPA/B CITE CMS name D S PROTO COL Pardela Annex II Calonectris cenicienta Pufin de ﺟَﻠَ ﻢ ﻣﺎ ء ﺳﻜﻮﺑﻮﻟﻲ diomedea mediterráne Scopoli a Pardela Puffin des ﺟﻠﻢ ﻣﺎء إﻧﺠﻠﯿﺰي Puffinus puffinus LATIN NAME ENGLISH ARABIC pichoneta Anglais MARINE MAMMALS Pardela Annex II ﺟﻠﻢ ﻣﺎء ﻣﺘﻮﺳﻄﻲ Puffinus yelkouan mediterráne Puffin yelkouan Balaenoptera physalus Fin whale a اﻟﺣوت اﻟﺷﺎﺋﻊ Puffinus Pardela Puffin des Annex II Appendix ﺟﻠﻢ ﻣﺎء ﻣﻮرﯾﻄﺎﻧﻲ Balaenoptera Common minke whale mauretanicus balear Baléares I (منكي حوت) القزم الحوت acutorostrata Alcatraz أطﯿﺶ ﺷﻤﺎﻟﻲ Morus bassanus Fou de Bassan Physeter macrocephalus Sperm whale atlántico MAMMALS SEABIRDS SEA TURTLES ﺣوت MARINEاﻟﻌﻧﺑر Cormoran Annex II Ziphius cavirostris Cuvier’s beaked whale Gulosus aristotelis Cormorán ﻏﺎق أرﺳﻄﻮ huppé (de زﯾﻔﯾوس (ﺣوت ﻛوﻓﯾﯾر اﻟﻣﻧﻘﺎري) desmarestii moñudo LATIN NAME ENGLISH Méditerranée) أرﻛﺔ (اﻟﺣوت اﻟﻘﺎﺗل) Orcinus orca Killer whale -

Paleogene Origin of Planktivory in the Batoidea
Paleogene Origin Of Planktivory In The Batoidea CHARLIE J. UNDERWOOD, 1+ MATTHEW A. KOLMANN, 2 and DAVID J. WARD 3 1Department of Earth and Planetary Sciences, Birkbeck, University of London, UK, [email protected]; 2 Department of Ecology and Evolutionary Biology, University of Toronto, Canada, [email protected]; 3Department of Earth Sciences, Natural History Museum, London, UK, [email protected] +Corresponding author RH: UNDERWOOD ET AL.—ORIGIN OF PLANKTIVOROUS BATOIDS 1 ABSTRACT—The planktivorous mobulid rays are a sister group to, and descended from, rhinopterid and myliobatid rays which possess a dentition showing adaptations consistent with a specialized durophageous diet. Within the Paleocene and Eocene there are several taxa which display dentitions apparently transitional between these extreme trophic modality, in particular the genus Burnhamia. The holotype of Burnhamia daviesi was studied through X-ray computed tomography (CT) scanning. Digital renderings of this incomplete but articulated jaw and dentition revealed previously unrecognized characters regarding the jaw cartilages and teeth. In addition, the genus Sulcidens gen. nov. is erected for articulated dentitions from the Paleocene previously assigned to Myliobatis. Phylogenetic analyses confirm Burnhamia as a sister taxon to the mobulids, and the Mobulidae as a sister group to Rhinoptera. Shared dental characters between Burnhamia and Sulcidens likely represent independent origins of planktivory within the rhinopterid – myliobatid clade. The transition from highly-specialized durophagous feeding morphologies to the morphology of planktivores is perplexing, but was facilitated by a pelagic swimming mode in these rays and we propose through subsequent transition from either meiofauna-feeding or pelagic fish-feeding to pelagic planktivory. -

National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE)
Project Code: MB5201 National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE) Authors: J. R. Ellis, V. A. Bendall, S. J. Hetherington, J. F. Silva and S. R. McCully Phillips. Issue date: 15-Jan-2016 Cefas Document Control Title: National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE) Project Code: MB5201 Submitted to: Defra Date submitted: 15-Jan-2016 Project Manager: Jim Ellis Project Sponsor: Wendy Dawson Report compiled by: Jim Ellis, Vicky Bendall, Stuart Hetherington, Joana Silva and Sophy McCully Phillips Quality control by: David Righton Approved by & date: Version: V1.4 Ellis, J. R., Bendall, V. A., Hetherington, S. J., Silva, J. F. and McCully Suggested citation Phillips, S. R. (2015). National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE). Project Report (Cefas), x + 105 pp. Version Control History Author Date Comment Version Ellis et al. 25 Feb 2015 Initial draft V1.0 Ellis et al. 26 Feb 2015 Edits made by JE and SM V1.1 Ellis et al. 04 Mar 2015 QA by DR, further edits by JE/SM V1.2 Ellis et al. 24 Jul 2015 Following comments from PSG V1.3 Ellis et al. 15 Jan 2016 Final version after external review V1.4 National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE) Page i National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE) Page ii Project Code: MB5201 National Evaluation of Populations of Threatened and Uncertain Elasmobranchs (NEPTUNE) Authors: J. R. Ellis, V. A. Bendall, -

Fish, Crustaceans, Molluscs, Etc Capture Production by Species
440 Fish, crustaceans, molluscs, etc Capture production by species items Atlantic, Northeast C-27 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Atlantique, nord-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Atlántico, nordeste English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2002 2003 2004 2005 2006 2007 2008 Nombre inglés Nombre científico Grupo de especies t t t t t t t Freshwater bream Abramis brama 11 2 023 1 650 1 693 1 322 1 240 1 271 1 299 Freshwater breams nei Abramis spp 11 1 543 1 380 1 412 1 420 1 643 1 624 1 617 Common carp Cyprinus carpio 11 11 4 2 - 0 - 1 Tench Tinca tinca 11 1 2 5 5 10 9 13 Crucian carp Carassius carassius 11 69 45 28 45 24 38 30 Roach Rutilus rutilus 11 4 392 3 630 3 467 3 334 3 409 3 571 3 339 Rudd Scardinius erythrophthalmus 11 2 1 - - - - - Orfe(=Ide) Leuciscus idus 11 211 216 164 152 220 220 233 Vimba bream Vimba vimba 11 277 149 122 129 84 99 97 Sichel Pelecus cultratus 11 523 532 463 393 254 380 372 Asp Aspius aspius 11 23 23 20 17 27 26 4 White bream Blicca bjoerkna 11 - - - - - 0 1 Cyprinids nei Cyprinidae 11 63 59 34 80 132 91 121 Northern pike Esox lucius 13 2 307 2 284 2 102 2 049 3 125 3 077 3 077 Wels(=Som)catfish Silurus glanis 13 - - 0 0 1 1 1 Burbot Lota lota 13 346 295 211 185 257 247 242 European perch Perca fluviatilis 13 5 552 6 012 5 213 5 460 6 737 6 563 6 122 Ruffe Gymnocephalus cernuus 13 31 - 2 1 2 2 1 Pike-perch Sander lucioperca 13 2 363 2 429 2 093 1 698 2 017 2 117 1 771 Freshwater -
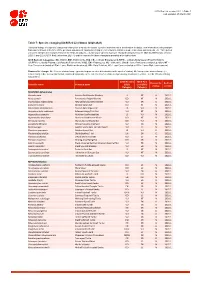
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Fish, Crustaceans, Molluscs, Etc Capture Production by Species
465 Fish, crustaceans, molluscs, etc Capture production by species items Atlantic, Northeast C-27 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Atlantique, nord-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Atlántico, nordeste English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2005 2006 2007 2008 2009 2010 2011 Nombre inglés Nombre científico Grupo de especies t t t t t t t Freshwater bream Abramis brama 11 1 322 1 240 1 271 1 386 1 691 1 608 1 657 Freshwater breams nei Abramis spp 11 1 420 1 643 1 624 1 617 1 705 1 628 1 869 Common carp Cyprinus carpio 11 - 0 - 1 0 2 2 Tench Tinca tinca 11 5 10 9 13 14 11 14 Crucian carp Carassius carassius 11 45 24 38 30 43 36 33 Roach Rutilus rutilus 11 3 334 3 409 3 571 2 935 2 957 2 420 2 662 Rudd Scardinius erythrophthalmus 11 - - - - - - 3 Orfe(=Ide) Leuciscus idus 11 152 220 220 268 262 71 83 Vimba bream Vimba vimba 11 129 84 99 97 93 91 116 Sichel Pelecus cultratus 11 393 254 380 372 417 312 423 Asp Aspius aspius 11 17 27 26 4 31 3 2 White bream Blicca bjoerkna 11 - - 0 1 1 23 70 Cyprinids nei Cyprinidae 11 80 132 91 121 162 45 94 Northern pike Esox lucius 13 2 049 3 125 3 077 1 915 1 902 1 753 1 838 Wels(=Som) catfish Silurus glanis 13 0 1 1 1 2 3 2 Burbot Lota lota 13 185 257 247 121 134 127 128 European perch Perca fluviatilis 13 5 460 6 737 6 563 5 286 5 145 5 072 5 149 Ruffe Gymnocephalus cernuus 13 1 2 2 1 1 33 61 Pike-perch Sander lucioperca 13 1 698 2 017 2 117 1 730 1 768 1 404 1 653 Freshwater -

Historical Biogeography of the Late Cretaceous Vertebrates of India: Comparison of Geophysical and Paleontological Data
Khosla, A. and Lucas, S.G., eds., 2016, Cretaceous Period: Biotic Diversity and Biogeography. New Mexico Museum of Natural History and Science Bulletin 71. 317 HISTORICAL BIOGEOGRAPHY OF THE LATE CRETACEOUS VERTEBRATES OF INDIA: COMPARISON OF GEOPHYSICAL AND PALEONTOLOGICAL DATA OMKAR VERMA1, ASHU KHOSLA2, FRANCISCO J. GOIN3 AND JASDEEP KAUR2 1Geology Discipline Group, School of Sciences, Indira Gandhi National Open University, New Delhi – 110 068, India, e-mail: omkarverma@ ignou.ac.in; 2Department of Geology, Centre for Advanced Studies, Panjab University, Sector-14, Chandigarh – 160014, India, e-mail: [email protected], e-mail: [email protected]; 3Consejo Nacional de Investigaciones Científicas y Técnicas and División Paleontología Vertebrados, Museo de Ciencias Naturales de La Plata, B1900FWA La Plata, Argentina, e-mail: [email protected] Abstract—The Cretaceous was a special time for the Indian plate as it was separated from Gondwana landmasses and started its northward journey across the Tethys Sea towards the Equator. The northward movement of this plate implied shifting latitudes and climate belts, until it finally collided with Asia during the early Cenozoic. Geophysical data and plate tectonic models show that after splitting from Gondwana, the Indian plate remained as an isolated continent for more than 45 Ma during the Cretaceous; thus, it predicts a remarkable biotic endemism for the continent. Paleontological data on the Cretaceous vertebrates of India is best known for Maastrichtian time; in turn, the pre-Maastrichtian record is very poor—it contains very few fossils of fishes and marine reptiles. The Maastrichtian fossil record comprises vertebrates of Gondwana and Laurasian affinities and some endemic, ancient lineages as well.