Catalytic Ring Opening of Decalin – Bifunctional Versus Hydrogenolytic Pathways J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decalin Dehydrogenation for In-Situ Hydrogen Production To
DECALIN DEHYDROGENATION FOR IN-SITU HYDROGEN PRODUCTION TO INCREASE CATALYTIC CRACKING RATE OF N-DODECANE Thesis Submitted to The School of Engineering of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Chemical Engineering By Christopher Bruening Dayton, Ohio May, 2018 DECALIN DEHYDROGENATION FOR IN-SITU HYDROGEN PRODUCTION TO INCREASE CATALYTIC CRACKING RATE OF N-DODECANE Name: Bruening, Christopher Robbins APPROVED BY: Matthew J. DeWitt, Ph.D. Donald K. Phelps, Ph.D. Advisory Committee Chairman Committee Member Distinguished Research Engineer Senior Research Chemist University of Dayton Research Institute Air Force Research Laboratory Michael Elsass, Ph.D. Kevin Myers, D.Sc., P.E. Committee Member Committee Member Lecturer Professor Department of Chemical and Materials Department of Chemical and Materials Engineering Engineering Robert J. Wilkens, Ph.D., P.E. Eddy M. Rojas, Ph.D., M.A., P.E. Associate Dean for Research and Innovation Dean, School of Engineering Professor School of Engineering School of Engineering ii ABSTRACT DECALIN DEHYDROGENATION FOR IN-SITU HYDROGEN PRODUCTION TO INCREASE CATALYTIC CRACKING RATE OF N-DODECANE Name: Bruening, Christopher Robbins University of Dayton Advisor: Dr. Matthew J. DeWitt Catalytic cracking of paraffinic hydrocarbons is a widely utilized industrial process, but catalyst deactivation over time requires regeneration or replacement of the catalyst bed. A gaseous hydrogen co-feed can be used to promote hydrocracking and decrease deactivation of the catalyst due to coke formation or active site poisoning. One potential alternative approach to extend the lifetime of a cracking catalyst is to generate molecular hydrogen in-situ via catalytic dehydrogenation of a cycloparaffin. -

Dibasic Acids for Nylon Manufacture
- e Report No. 75 DIBASIC ACIDS FOR NYLON MANUFACTURE by YEN-CHEN YEN October 1971 A private report by the PROCESS ECONOMICS PROGRAM STANFORD RESEARCH INSTITUTE MENLO PARK, CALIFORNIA CONTENTS INTRODUCTION, ....................... 1 SUMMARY .......................... 3 General Aspects ...................... 3 Technical Aspects ..................... 7 INDUSTRY STATUS ...................... 15 Applications and Consumption of Sebacic Acid ........ 15 Applications and Consumption of Azelaic Acid ........ 16 Applications of Dodecanedioic and Suberic Acids ...... 16 Applications of Cyclododecatriene and Cyclooctadiene .... 17 Producers ......................... 17 Prices ........................... 18 DIBASIC ACIDS FOR MANUFACTURE OF POLYAMIDES ........ 21 CYCLOOLIGOMERIZATIONOF BUTADIENE ............. 29 Chemistry ......................... 29 Ziegler Catalyst ..................... 30 Nickel Catalyst ..................... 33 Other Catalysts ..................... 34 Co-Cyclooligomerization ................. 34 Mechanism ........................ 35 By-products and Impurities ................ 37 Review of Processes .................... 38 A Process for Manufacture of Cyclododecatriene ....... 54 Process Description ................... 54 Process Discussion .................... 60 Cost Estimates ...................... 60 A Process for Manufacture of Cyclooctadiene ........ 65 Process Description ................... 65 Process Discussion .................... 70 Cost Estimates ...................... 70 A Process for Manufacture of Cyclodecadiene -

Organic Contaminant Analytical Methods of the National Status and Trends Program: 2000-2006
Organic Contaminant Analytical Methods of the National Status and Trends Program: 2000-2006 NOAA Technical Memorandum NOS NCCOS 30 Mention of trade names or commercial products does not constitute endorsement or recommendation for their use by the United States government. Cover photograph of shrimping fleet, Palacios, TX Citation for this Report Kimbrough, K. L., G. G. Lauenstein and W. E. Johnson (Editors). 2006. Organic Contaminant Analytical Methods of the National Status and Trends Program: Update 2000-2006. NOAA Technical Memorandum NOS NCCOS 30. 137 pp. Organic Contaminant Analytical Methods of the National Status and Trends Program: Update 2000-2006 K. L. Kimbrough, G. G. Lauenstein and W. E. Johnson (Editors) Center for Coastal Monitoring and Assessment NOAA/NOS/NCCOS 1305 East-West Highway Silver Spring, Maryland 20910 NOAA Technical Memorandum NOS NCCOS 30 June 2007 United States Department of National Oceanic and National Ocean Service Commerce Atmospheric Administration Carlos M. Gutierrez Conrad C. Lautenbacher, Jr. John H. Dunnigan Secretary Administrator Assistant Administrator TABLE OF CONTENTS TABLE OF CONTENTS ..................................................................................................I LIST OF TABLES ..........................................................................................................III LIST OF FIGURES ........................................................................................................IV CHAPTER 1. INTRODUCTION................................................................................... -

Research on Crystal Growth and Characterization at the National Bureau of Standards January to June 1964
NATL INST. OF STAND & TECH R.I.C AlllDS bnSflb *^,; National Bureau of Standards Library^ 1*H^W. Bldg Reference book not to be '^sn^ t-i/or, from the library. ^ecknlccil v2ote 251 RESEARCH ON CRYSTAL GROWTH AND CHARACTERIZATION AT THE NATIONAL BUREAU OF STANDARDS JANUARY TO JUNE 1964 U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS tiona! Bureau of Standards NOV 1 4 1968 151G71 THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assuring maximum application of the physical and engineering sciences to the advancement of technology in industry and commerce. Its responsibilities include development and maintenance of the national stand- ards of measurement, and the provisions of means for making measurements consistent with those standards; determination of physical constants and properties of materials; development of methods for testing materials, mechanisms, and structures, and making such tests as may be necessary, particu- larly for government agencies; cooperation in the establishment of standard practices for incorpora- tion in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; assistance to industry, business, and consumers in the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation with United States business groups and standards organizations for the development of international standards of practice; and maintenance of a clearinghouse for the collection and dissemination of scientific, tech- nical, and engineering information. The scope of the Bureau's activities is suggested in the following listing of its four Institutes and their organizational units. -

United States Patent Office Patented
2,826,604 United States Patent Office Patented. Mar. 11, 1958 2 (130-140 C.), the decalyl acetate being recoverable from the reaction mixture by distillation of the neutralized re 2,826,604 action liquid. The recovered product was a straw-colored PREPARATION OF DECAHYDRONAPHTHYL fragrant oil, which was established to be 1-decallyl acetate. ACETATE 5 The position of the acetoxy group was determined by William E. Erner, Wilmington, Del, assignor to Houdry hydrolysis of the ester to the alcohol and subsequent Process Corporation, Wilmington, Del, a corporation conversion to decalone-1 by chromic acid-acetic acid oxi of Delaware dation. - The aceto-oxidation of Decalin can be carried out No Drawing. Application June 2, 1955 10 in the presence of oxidation catalysts of the type em Serial No. 512,853 bodied in the metal salts of the lower fatty acids. Cobalt 2 Claims. (C. 260-488) acetate proved outstanding among the metallic-soap oxi dation catalysts studied. The use of benzoyl peroxide at the start of the process has been found advantageous The present invention relates to the preparation of 1 5 in decreasing the induction period and in giving uni decahydronaphthyl alcohols and the corresponding acetic formly high yields. acid ester and ketone derivatives of Said alcohols. The ratios of Decalin - to acetic anhydride in the oper More particularly the invention is concerned with the ation of the process are not critical, but practical con production of saturated polynuclear carbocyclic com siderations favor the use of at least one-half mol of pounds of the type 20 acetic anhydride per mol of Decalin. -

Decalin Dissolving Method for Recover of Styrene –Butadiene Rubber from Scrap Tires
American Journal of Environmental Sciences Original Research Paper Decalin Dissolving Method for Recover of Styrene –Butadiene Rubber from Scrap Tires Aeppa M.T. Sultan and Fawzi Habeeb Jabrail Polymer Research Lab., Department of Chemistry, College of Science, University of Mosul, Iraq Article history Abstract: Decalin, a hydrocarbon industrial solvent was used for the Received: 18-08-2019 recovery of Styrene- Butadiene Rubber (SBR) from grind scrap truck tires Revised: 10-09-2019 using chemical dissolving method. The applied technique is easy, simple Accepted: 11-10-2019 and depends on available materials. The method depends mainly on Corresponding Author: steeping the scrap tires grind into decalin at 50C for one month. Then the Fawzi Habeeb Jabrail process was followed by boiling of the formed thick black solution Polymer Research Lab., (198°C) for two hours. The formed elastic rubber material will be Department of Chemistry, reclaimed by precipitation in methanol. The recovered rubber was College of Science, University characterized using FTIR spectroscopy and XRD analyses. The pattern of of Mosul, Iraq the recovered material shows broad maxima of SBR with the absence of Tel: +9647703336282 XRD peaks of carbon black and almost all the other tire manufacturing E-mail: [email protected] additives. The thermal characteristics of the reclaimed rubber have been investigated by TGA, DTG and DSC analyses and were found to be close to those of thermal properties of SBR polymer. The SEM image has shown non-crystalline morphological surface of the recovered rubber with cohesive elastomeric properties. Keywords: SBR Reclaims, Decalin Dissolving Method, Scrap Tires, Steeping Method, Recycling Introduction Vehicle tires are made of rubber after vulcanization with sulfur to render it crosslinked and after adding The world consumes 4.5 million tons of tires, in several additives, like fillers, steel wires, plasticizers, addition to billions of tires of different types are to be extenders accelerators, curing agents and others in order to disposed of in the USA. -

CORINA Classic Program Manual
CORINA Classic Generation of Three-Dimensional Molecular Models Version 4.4.0 Program Description Molecular Networks GmbH September 2021 www.mn-am.com Molecular Networks GmbH Altamira LLC Neumeyerstraße 28 470 W Broad St, Unit #5007 90411 Nuremberg Columbus, OH 43215 Germany USA mn-am.com This document is copyright © 1998-2021 by Molecular Networks GmbH Computerchemie and Altamira LLC. All rights reserved. Except as permitted under the terms of the Software Licensing Agreement of Molecular Networks GmbH Computerchemie, no part of this publication may be reproduced or distributed in any form or by any means or stored in a database retrieval system without the prior written permission of Molecular Networks GmbH or Altamira LLC. The software described in this document is furnished under a license and may be used and copied only in accordance with the terms of such license. (Document version: 4.4.0-2021-09-30) Content Content 1 Introducing CORINA Classic 1 1.1 Objective of CORINA Classic 1 1.2 CORINA Classic in Brief 1 2 Release Notes 3 2.1 CORINA (Full Version) 3 2.2 CORINA_F (Restricted FlexX Interface Version) 25 3 Getting Started with CORINA Classic 26 4 Using CORINA Classic 29 4.1 Synopsis 29 4.2 Options 29 5 Use Cases of CORINA Classic 60 6 Supported File Formats and Interfaces 64 6.1 V2000 Structure Data File (SD) and Reaction Data File (RD) 64 6.2 V3000 Structure Data File (SD) and Reaction Data File (RD) 67 6.3 SMILES Linear Notation 67 6.4 InChI file format 69 6.5 SYBYL File Formats 70 6.6 Brookhaven Protein Data Bank Format (PDB) -

Addition Reactions and Photoisomerization of Cis,Trans-1,5-Cyclodecadiene
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1970 Addition Reactions and Photoisomerization of Cis,trans-1,5-Cyclodecadiene. Hsin-hsiong Hsieh Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Hsieh, Hsin-hsiong, "Addition Reactions and Photoisomerization of Cis,trans-1,5-Cyclodecadiene." (1970). LSU Historical Dissertations and Theses. 1859. https://digitalcommons.lsu.edu/gradschool_disstheses/1859 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. 71-6579 HSIEH, Hsin-Hsiong, 1936- ADDITION REACTIONS AND PHOTOISOMERIZATION OF CIS,TRANS-1,5-CYCLODECADIENE. The Louisiana State University and Agricultural and Mechanical College, Ph.D., 1970 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan ADDITION REACTIONS AND PHOTOISOMERIZATION OF CIS,TRANS-1,5“CYCLODECADIENE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Chemistry by Hsin-Hsiong Hsieh B.S., Taiwan Provincial Chung-Hsing University, i960 M.S., Louisiana State University, -

Forensic Testing Capabilities
Forensic Testing Capabilities Understanding what type of fuel you’re dealing Fuel Type Carbon Range with at your site can be critical in determining your best course of corrective action. Eurofins Gasoline* (semivolatile portion only with C8 – C12 Lancaster Laboratories Environmental can extractables TPH; semi-quantitative) provide you with the analysis and expert interpretation of your samples with unknown Stoddard Solvent* petroleum products. (also referred to as mineral C9 – C12 spirits, VM&P naphtha) Fingerprint TPH by GC/FID Kerosene and Jet Fuels* C – C We analyze samples using GC/FID Method 9 19 SW-846 8015B from C8-C40 to identify the #2 Fuel/Diesel* C – C extractable petroleum products in soil, water 10 28 and natural fuels and compare your sample #6 Fuel* chromatogram against our petroleum standard (also referred to as C10 – C40 reference library. bunker or residual fuel) PAHs, Alkylated PAHs and Biomarker Motor Oil* C16 – C36 Analysis (SW-846 8270 SIM) Hydraulic Oil C – C Some of the most important target analytes in 20 40 damage assessment from petroleum products C – C are PAHs and alkylated PAHs. Coal Tar Oil* 10 36 They can also be used in forensic analyses *Indicates analysis with reference standards. when presented as histograms that visually Eurofins Lancaster Laboratories represent the relative target analyte Environmental provides analysis of water, concentrations for the purpose of qualitatively soil, sediment, product and tissue samples fingerprinting a petroleum sample. Crude oils for alkanes, TPH/SHC/DRO and isoprenoids contain primarily Alkylated PAHs and relatively using GC/FID SW-846 8015, including: small concentrations of the unsubstituted parent PAHs. -

Cal/OSHA, DOT HAZMAT, EEOC, EPA, HIPAA, IATA, IMDG, TDG, MSHA, OSHA, Australia WHS, and Canada OHS Regulations and Safety Online Training
! Cal/OSHA, DOT HAZMAT, EEOC, EPA, HIPAA, IATA, IMDG, TDG, MSHA, OSHA, Australia WHS, and Canada OHS Regulations and Safety Online Training This document is provided as a training aid and may not reflect current laws and regulations. Be sure and consult with the appropriate governing agencies or publication providers listed in the "Resources" section of our website. www.ComplianceTrainingOnline.com ! ! ! ! ! Facebook LinkedIn Twitter Google Plus Website Hazardous Chemicals Handbook Second edition Phillip Carson PhD MSc AMCT CChem FRSC FIOSH Head of Science Support Services, Unilever Research Laboratory, Port Sunlight, UK Clive Mumford BSc PhD DSc CEng MIChemE Consultant Chemical Engineer Oxford Amsterdam Boston London New York Paris San Diego San Francisco Singapore Sydney Tokyo Butterworth-Heinemann An imprint of Elsevier Science Linacre House, Jordan Hill, Oxford OX2 8DP 225 Wildwood Avenue, Woburn, MA 01801-2041 First published 1994 Second edition 2002 Copyright © 1994, 2002, Phillip Carson, Clive Mumford. All rights reserved The right of Phillip Carson and Clive Mumford to be identified as the authors of this work has been asserted in accordance with the Copyright, Designs and Patents Act 1988 No part of this publication may be reproduced in any material form (including photocopying or storing in any medium by electronic means and whether or not transiently or incidentally to some other use of this publication) without the written permission of the copyright holder except in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of a licence issued by the Copyright Licensing Agency Ltd, 90 Tottenham Court Road, London, England W1T 4LP. -

2002-00238-01-E.Pdf (Pdf)
report no. 01/54 environmental classification of petroleum substances - summary data and rationale Prepared by CONCAWE Petroleum Products Ecology Group D. King (Chairman) M. Bradfield P. Falkenback T. Parkerton D. Peterson E. Remy R. Toy M. Wright B. Dmytrasz D. Short Reproduction permitted with due acknowledgement CONCAWE Brussels October 2001 I report no. 01/54 ABSTRACT Environmental data on the fate and effects of petroleum substances are summarised. Technical issues relating to the choice of test methodology for the evaluation of environmental impacts of petroleum substances are discussed. Proposals for self-classification according to EU criteria, as defined in the Dangerous Substances Directive, are presented for individual petroleum substance groups, on the basis of available test data and structure activity relationships (based on composition). KEYWORDS Hazard, environment, petroleum substances, classification, aquatic toxicity, biodegradation, bioaccumulation, QSAR, dangerous substances directive. INTERNET This report is available as an Adobe pdf file on the CONCAWE website (www.concawe.be). NOTE Considerable efforts have been made to assure the accuracy and reliability of the information contained in this publication. However, neither CONCAWE nor any company participating in CONCAWE can accept liability for any loss, damage or injury whatsoever resulting from the use of this information. This report does not necessarily represent the views of any company participating in CONCAWE. II report no. 01/54 CONTENTS Page SUMMARY V 1. REGULATORY FRAMEWORK AND GENERAL INTRODUCTION 1 2. DATA SELECTION FOR CLASSIFICATION 2 2.1. WATER "SOLUBILITY" OF PETROLEUM SUBSTANCES AND TEST "CONCENTRATIONS" 2 2.2. ACUTE AND CHRONIC TOXICITY AND SOLUBILITY LIMITS 2 2.3. PERSISTENCE AND FATE PROCESSES FOR PETROLEUM SUBSTANCES 3 2.4. -
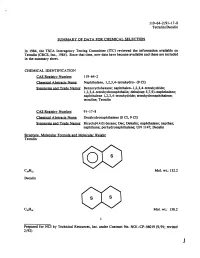
Tetralin/Decalin
119-64-2/91-17-8 Tetralin/Decalin SUMMARY OF DATA FOR CHEMICAL SELECTION In 1984, the TSCA Interagency Testing Committee (lTC) reviewed the information available on Tetralin (CRCS, Inc., 1984). Since that time, new data have become available and these are included in the summary sheet. CHEMICAL IDENTIFICATION CAS Registry Number: 119-64-2 Chemical Abstracts Name: Naphthalene, 1,2,3,4-tetrahydro- (9 CI) Synonyms and Trade Names: Benzocyclohexane; naphthalen-1,2,3,4-tetrahydride; 1,2,3,4-tetrahydronaphthalin; delta(sup 5,7 ,9)-naphthalene; naphthalene 1,2,3,4-tetrahydride; tetrahydronaphthalene; tetraline; Tetralin CAS Registry Number: 91-17-8 Chemical Abstracts Name: Decahydronaphthalene (8 CI, 9 CI) Synonyms and Trade Names: Bicyc1o[4.4.0] decane; Dec; Dekalin; naphthalane; napthan; naphthane; perhydronaphthalene; UN 1147; Decalin Structure. Molecular Formula and Molecular Weight Tetralin Mol. wt.: 132.2 Decal in Mol. wt.: 138.2 1 Prepared for NCI by Technical Resources, Inc. under Contract No. N01-CP-56019 (9/91; revised 2/92) 1 119-64-2/91-17-8 Tetralin/Decalin Chemical and Physical Prooerties: From Budavari (1989) unless otherwise noted. Tetralin Description: Liquid with odor resembling that of a mixture of benzene and menthol Boiling Point: 207.2° C Melting Point: -31 o C Solubility: Insoluble in water; soluble in methanol at 50.6% wtjwt and aniline; very soluble in ether; miscible with petroleum ether, chloroform, and Decalin, ethanol, butanol, acetone and benzene StabilitY: Prolonged, intimate contact with air may cause the formation