Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Rare Missense Mutation in GJB3 (Cx31g45e) Is Associated with a Unique Cellular Phenotype Resulting in Necrotic Cell Death Easton, J
University of Dundee A rare missense mutation in GJB3 (Cx31G45E) is associated with a unique cellular phenotype resulting in necrotic cell death Easton, J. A.; Alboulshi, A. K.; Kamps, M. A. F.; Brouns, G. H.; Broers, M. R.; Coull, B. J.; Oji, V.; van Geel, M.; van Steensel, M. A. M.; Martin, P. E. Published in: Experimental Dermatology DOI: 10.1111/exd.13542 Publication date: 2018 Document Version Peer reviewed version Link to publication in Discovery Research Portal Citation for published version (APA): Easton, J. A., Alboulshi, A. K., Kamps, M. A. F., Brouns, G. H., Broers, M. R., Coull, B. J., ... Martin, P. E. (2018). A rare missense mutation in GJB3 (Cx31G45E) is associated with a unique cellular phenotype resulting in necrotic cell death. Experimental Dermatology. https://doi.org/10.1111/exd.13542 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

A Computational Model of Large Conductance Voltage and Calcium
Journal of Computational Neuroscience (2019) 46:233–256 https://doi.org/10.1007/s10827-019-00713-9 A computational model of large conductance voltage and calcium activated potassium channels: implications for calcium dynamics and electrophysiology in detrusor smooth muscle cells Suranjana Gupta1 · Rohit Manchanda1 Received: 11 September 2018 / Revised: 14 February 2019 / Accepted: 19 February 2019 / Published online: 25 April 2019 © Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract The large conductance voltage and calcium activated potassium (BK) channels play a crucial role in regulating the excitability of detrusor smooth muscle, which lines the wall of the urinary bladder. These channels have been widely characterized in terms of their molecular structure, pharmacology and electrophysiology. They control the repolarising and hyperpolarising phases of the action potential, thereby regulating the firing frequency and contraction profiles of the smooth muscle. Several groups have reported varied profiles of BK currents and I-V curves under similar experimental conditions. However, no single computational model has been able to reconcile these apparent discrepancies. In view of the channels’ physiological importance, it is imperative to understand their mechanistic underpinnings so that a realistic model can be created. This paper presents a computational model of the BK channel, based on the Hodgkin-Huxley formalism, constructed by utilising three activation processes — membrane potential, calcium inflow from voltage-gated calcium channels on the membrane and calcium released from the ryanodine receptors present on the sarcoplasmic reticulum. In our model, we attribute the discrepant profiles to the underlying cytosolic calcium received by the channel during its activation. The model enables us to make heuristic predictions regarding the nature of the sub-membrane calcium dynamics underlying the BK channel’s activation. -
ION CHANNEL DIVERSITY and CHARACTERIZATION
ION CHANNEL DIVERSITY and CHARACTERIZATION Voltage clamp techniques K channels Na channels Ca channels Ligand-gated channels Channelopathies OUTLINE Voltage clamp techniques whole cell, single channel, gating K channels Na channels Ca channels Cardiac AP Na nerve vs cardiac Ica: L vs T type; drugs (BayK, nitrendipine) Ito: inactivation, subtypes Kv1.4, Kv4.2/3, accessory subunits Ikr,Iks, Ikur (drugs dofetilide) IK1 – rectification Cardiac channelopathies (LQTS, SQTS, Brugada syndrome) Ligand-gated channels (AChR) Pancreatic beta cell channels (KATP, ICa) Voltage clamp techniques Capacitance currents (Ic) and ionic currents (Ii) are activated by rapid changes in membrane potential using voltage clamp Variable Vtest can be applied with voltage clamp 2.0 sec 40 duration 20 (10 sec between each pulse) 0 -20 mV test -40 V -60 -80 Simplified schematic of voltage clamp circuit Original patch clamp recordings (1981) Pflugers Arch 391: 85-100 Four modes of patch clamp technique High-throughput, automated patch clamp instruments EVOLUTION and Ion channel diversity Diversity of ion channels Example: nematode C. elegans 73 K channels (20 6 TM, 3 IRK, 50 TWIK) 89 ligand-gated channels (42 ACh, 37 inhibitory GABAA or glutamate, 10 excitatory glutamate) 5 voltage-gated Ca channels 6 chloride channels 24 gap junction channels (connexins) 22 mechanosensitive channels 6 cyclic-nucleotide gated channels 11 TRP-related channels Total: 236 channel subunit genes Origin of ion channel diversity 1) gene duplication & divergence 2) alternative mRNA splicing 3) -

GJA4/Connexin 37 Mutations Correlate with Secondary Lymphedema Following Surgery in Breast Cancer Patients
biomedicines Article GJA4/Connexin 37 Mutations Correlate with Secondary Lymphedema Following Surgery in Breast Cancer Patients Mahrooyeh Hadizadeh 1,2, Seiied Mojtaba Mohaddes Ardebili 1, Mansoor Salehi 2, Chris Young 3, Fariborz Mokarian 4, James McClellan 5, Qin Xu 6, Mohammad Kazemi 2, Elham Moazam 4, Behzad Mahaki 7 ID and Maziar Ashrafian Bonab 8,* 1 Department of Medical Genetics, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz 5166614766, Iran; [email protected] (M.H.); [email protected] (S.M.M.A.) 2 Department of Genetics and Molecular Biology, Isfahan University of Medical Sciences, Isfahan 81746753461, Iran; [email protected] (M.S.); [email protected] (M.K.) 3 School of Allied Health Sciences, Faculty of Health and Life Sciences, De Montfort University, Leicester LE1 9BH, UK; [email protected] 4 Cancer Prevention Research Centre, Isfahan University of Medical Sciences, Isfahan 8184917911, Iran; [email protected] (F.M.); [email protected] (E.M.) 5 School of Biological Sciences, University of Portsmouth, Portsmouth PO1 2DY, UK; [email protected] 6 School of Pharmacy, Faculty of Health and Life Sciences, De Montfort University, Leicester LE1 9BH, UK; [email protected] 7 Department of Occupational Health Engineering, School of Health, Isfahan University of Medical Sciences, Isfahan 8174673461, Iran; [email protected] 8 Department of Biological Sciences, University of Chester, Chester CH1 4BJ, UK * Correspondence: [email protected]; Tel.: +44-(0)1244-513-056 Received: 31 December 2017; Accepted: 13 February 2018; Published: 22 February 2018 Abstract: Lymphedema is a condition resulting from mutations in various genes essential for lymphatic development and function, which leads to obstruction of the lymphatic system. -

Cell-Cell Interactions
7 Cell-Cell Interactions Concept Outline 7.1 Cells signal one another with chemicals. Receptor Proteins and Signaling between Cells. Receptor proteins embedded in the plasma membrane change shape when they bind specific signal molecules, triggering a chain of events within the cell. Types of Cell Signaling. Cell signaling can occur between adjacent cells, although chemical signals called hormones act over long distances. 7.2 Proteins in the cell and on its surface receive signals from other cells. Intracellular Receptors. Some receptors are located within the cell cytoplasm. These receptors respond to lipid- soluble signals, such as steroid hormones. Cell Surface Receptors. Many cell-to-cell signals are water-soluble and cannot penetrate membranes. Instead, the signals are received by transmembrane proteins protruding out from the cell surface. 7.3 Follow the journey of information into the cell. FIGURE 7.1 Persimmon cells in close contact with one another. These Initiating the Intracellular Signal. Cell surface receptors plant cells and all cells, no matter what their function, interact often use “second messengers” to transmit a signal to the with their environment, including the cells around them. cytoplasm. Amplifying the Signal: Protein Kinase Cascades. Surface receptors and second messengers amplify signals as id you know that each of the 100 trillion cells of your they travel into the cell, often toward the cell nucleus. Dbody shares one key feature with the cells of tigers, bumblebees, and persimmons (figure 7.1)—a feature that 7.4 Cell surface proteins mediate cell-cell interactions. most bacteria and protists lack? Your cells touch and com- The Expression of Cell Identity. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover
biomolecules Article Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover Joell L. Solan 1 and Paul D. Lampe 1,2,* 1 Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA; [email protected] 2 Department of Global Health, Pathobiology Program, University of Washington, Seattle, WA 98109, USA * Correspondence: [email protected] Received: 27 October 2020; Accepted: 22 November 2020; Published: 24 November 2020 Abstract: The gap junction protein Connexin43 (Cx43) is highly regulated by phosphorylation at over a dozen sites by probably at least as many kinases. This Cx43 “kinome” plays an important role in gap junction assembly and turnover. We sought to gain a better understanding of the interrelationship of these phosphorylation events particularly related to src activation and Cx43 turnover. Using state-of-the-art live imaging methods, specific inhibitors and many phosphorylation-status specific antibodies, we found phospho-specific domains in gap junction plaques and show evidence that multiple pathways of disassembly exist and can be regulated at the cellular and subcellular level. We found Src activation promotes formation of connexisomes (internalized gap junctions) in a process involving ERK-mediated phosphorylation of S279/282. Proteasome inhibition dramatically and rapidly restored gap junctions in the presence of Src and led to dramatic changes in the Cx43 phospho-profile including to increased Y247, Y265, S279/282, S365, and S373 phosphorylation. Lysosomal inhibition, on the other hand, nearly eliminated phosphorylation on Y247 and Y265 and reduced S368 and S373 while increasing S279/282 phosphorylation levels. We present a model of gap junction disassembly where multiple modes of disassembly are regulated by phosphorylation and can have differential effects on cellular signaling. -

Entropy-Based Regulation of Cluster Ion Channel Density
www.nature.com/scientificreports OPEN Molecular and cellular correlates in Kv channel clustering: entropy‑based regulation of cluster ion channel density Limor Lewin1, Esraa Nsasra1, Ella Golbary1, Uzi Hadad2, Irit Orr1 & Ofer Yifrach1* Scafold protein-mediated ion channel clustering at unique membrane sites is important for electrical signaling. Yet, the mechanism(s) by which scafold protein-ion channel interactions lead to channel clustering or how cluster ion channel density is regulated is mostly not known. The voltage‑activated potassium channel (Kv) represents an excellent model to address these questions as the mechanism underlying its interaction with the post-synaptic density 95 (PSD-95) scafold protein is known to be controlled by the length of the extended ‘ball and chain’ sequence comprising the C-terminal channel region. Here, using sub-difraction high-resolution imaging microscopy, we show that Kv channel ‘chain’ length regulates Kv channel density with a ‘bell’-shaped dependence, refecting a balance between thermodynamic considerations controlling ‘chain’ recruitment by PSD-95 and steric hindrance due to the spatial proximity of multiple channel molecules. Our results thus reveal an entropy‑based mode of channel cluster density regulation that mirrors the entropy‑based regulation of the Kv channel-PSD-95 interaction. The implications of these fndings for electrical signaling are discussed. Action potential generation, propagation and the evoked synaptic potential all rely on precisely timed events associated with activation and inactivation gating transitions of voltage-dependent Na + and K + channels, clus- tered in multiple copies at unique membrane sites, such as the initial segment of an axon, nodes of Ranvier, pre-synaptic terminals or at the post-synaptic density (PSD)1–3. -

Cooperative Coupling of Cell-Matrix and Cell–Cell Adhesions in Cardiac Muscle
Cooperative coupling of cell-matrix and cell–cell adhesions in cardiac muscle Megan L. McCaina, Hyungsuk Leea,1, Yvonne Aratyn-Schausa, André G. Kléberb, and Kevin Kit Parkera,2 aDisease Biophysics Group, Wyss Institute for Biologically Inspired Engineering, School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138; and bDepartment of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02215 Edited by Robert Langer, Massachusetts Institute of Technology, Cambridge, MA, and approved May 1, 2012 (received for review February 21, 2012) Adhesion between cardiac myocytes is essential for the heart to serve as cues for remodeling adhesions and assembling tissues. In function as an electromechanical syncytium. Although cell-matrix vitro studies have demonstrated that cytoskeletal tension (27) and and cell–cell adhesions reorganize during development and dis- exogenous cyclic strain (28, 29) promote cell–cell adhesion and ease, the hierarchical cooperation between these subcellular struc- tissue assembly in many cell types. Culturing noncardiac cells tures is poorly understood. We reasoned that, during cardiac on stiff substrates tips the balance of adhesion in favor of focal development, focal adhesions mechanically stabilize cells and tis- adhesions and away from cell–cell adhesions (30–32), suggesting sues during myofibrillogenesis and intercalated disc assembly. As that mechanical forces can modulate the assembly or disassembly the intercalated disc matures, we postulated that focal -

Localized Calcium Signaling and the Control of Coupling at Cx36 Gap Junctions
Research Article: New Research | Novel Tools and Methods Localized calcium signaling and the control of coupling at Cx36 gap junctions https://doi.org/10.1523/ENEURO.0445-19.2020 Cite as: eNeuro 2020; 10.1523/ENEURO.0445-19.2020 Received: 25 October 2019 Revised: 29 January 2020 Accepted: 21 February 2020 This Early Release article has been peer-reviewed and accepted, but has not been through the composition and copyediting processes. The final version may differ slightly in style or formatting and will contain links to any extended data. Alerts: Sign up at www.eneuro.org/alerts to receive customized email alerts when the fully formatted version of this article is published. Copyright © 2020 Moore et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International license, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed. 1 Localized calcium signaling and the control of coupling at Cx36 gap junctions 2 3 Abbreviated title: Calcium signaling at Cx36 gap junctions 4 5 Keith B. Moore1,†, Cheryl K. Mitchell1, Ya-Ping Lin1, Yuan-Hao Lee1, Eyad Shihabeddin1,2, and 6 John O'Brien1,2,* 7 8 1. Richard S. Ruiz, M.D. Department of Ophthalmology & Visual Science, McGovern Medical 9 School, The University of Texas Health Science Center at Houston, Houston, Texas, USA. 10 2. The MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, 11 Houston, Texas, USA. 12 †. Current Address: School of Public Health, The University of Texas Health Science Center at 13 Houston, Houston, Texas, USA. -
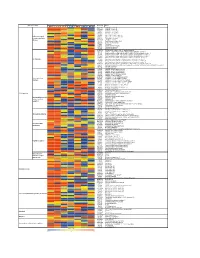
Supporting Figure 2
Normalized value Functional class Genbank Name Naive SC 1h SC 6h SC 24h WM 1h WM 6h WM 24h AB016161 GABA-B receptor 1d AF109405 GABA-B receptor 2a M35077 Dopamine-1A receptor S46131 Dopamine-1A receptor M84009 Dopamine receptor D4 U13368 Adrenergic receptor, alpha 1a G Protein-coupled M60654 Adrenergic receptor, alpha 1d receptors and their M64236 Tachykinin 1 receptor effectors AI229237 Opioid receptor-like Y11433 Pyrimidinergic receptor P2Y4 M64299 Adenosine A1 receptor E00001 Pro-insulin M29014 Insulin receptor precursor U35315 Serotonin receptor 2C S62043 Serotonin receptor 6 AF000368 Scn9a sodium channel, type IX, alpha polypeptide M27158 Kcna5 K+ voltage-gated channel, shaker-related subfamily, member 5 X17621 Kcna6 potassium voltage-gated channel, shaker-related, subfamily, member 6 X16476 Kcnb1 potassium voltage gated channel, Shab-related subfamily, member 1 M77482 Kcnb2 potassium voltage gated channel, Shab-related subfamily, member 2 S64320 Kcnd2 potassium voltage gated channel, Shal-related family, member 2 Ion channels X87635 Kcnj4 potassium inwardly-rectifying channel, subfamily J, member 4 D86039 Kcnj11 potassium inwardly-rectifying channel, subfamily J, member 11 X83581 Kcnj16 potassium inwardly-rectifying channel, subfamily J, member 16 AF073891 Kcnh5 potassium voltage-gated channel, subfamily H (eag-related), member 5 U69882 Kcnn2 potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 Z36944 Chloride channel 4-2 Z56277 Chloride channel 5 L08493 GABA-A receptor alpha-4 subunit X51992 -

Modeling of Voltage-Gated Ion Channels
Modeling of voltage-gated ion channels Modeling of voltage-gated ion channels Pär Bjelkmar c Pär Bjelkmar, Stockholm 2011, pages 1-65 Cover picture: Produced by Pär Bjelkmar and Jyrki Hokkanen at CSC - IT Center for Science, Finland. Cover of PLoS Computational Biology February 2009 issue. ISBN 978-91-7447-336-0 Printed in Sweden by US-AB, Stockholm 2011 Distributor: Department of Biochemistry and Biophysics, Stockholm University Abstract The recent determination of several crystal structures of voltage-gated ion channels has catalyzed computational efforts of studying these re- markable molecular machines that are able to conduct ions across bi- ological membranes at extremely high rates without compromising the ion selectivity. Starting from the open crystal structures, we have studied the gating mechanism of these channels by molecular modeling techniques. Firstly, by applying a membrane potential, initial stages of the closing of the channel were captured, manifested in a secondary-structure change in the voltage-sensor. In a follow-up study, we found that the energetic cost of translocating this 310-helix was significantly lower than in the origi- nal conformation. Thirdly, collaborators of ours identified new molecular constraints for different states along the gating pathway. We used those to build new protein models that were evaluated by simulations. All these results point to a gating mechanism where the S4 helix undergoes a secondary structure transformation during gating. These simulations also provide information about how the protein in- teracts with the surrounding membrane. In particular, we found that lipid molecules close to the protein diffuse together with it, forming a large dynamic lipid-protein cluster.