How to Integrate Planck's Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Lecture 26
PHYS 445 Lecture 26 - Black-body radiation I 26 - 1 Lecture 26 - Black-body radiation I What's Important: · number density · energy density Text: Reif In our previous discussion of photons, we established that the mean number of photons with energy i is 1 n = (26.1) i eß i - 1 Here, the questions that we want to address about photons are: · what is their number density · what is their energy density · what is their pressure? Number density n Eq. (26.1) is written in the language of discrete states. The first thing we need to do is replace the sum by an integral over photon momentum states: 3 S i ® ò d p Of course, it isn't quite this simple because the density-of-states issue that we introduced before: for every spin state there is one phase space state for every h 3, so the proper replacement more like 3 3 3 S i ® (1/h ) ò d p ò d r The units now work: the left hand side is a number, and so is the right hand side. But this expression ignores spin - it just deals with the states in phase space. For every photon momentum, there are two ways of arranging its polarization (i.e., the orientation of its electric or magnetic field vectors): where the photon momentum vector is perpendicular to the plane. Thus, we have 3 3 3 S i ® (2/h ) ò d p ò d r. (26.2) Assuming that our system is spatially uniform, the position integral can be replaced by the volume ò d 3r = V. -

Lecture 17 : the Cosmic Microwave Background
Let’s think about the early Universe… Lecture 17 : The Cosmic ! From Hubble’s observations, we know the Universe is Microwave Background expanding ! This can be understood theoretically in terms of solutions of GR equations !Discovery of the Cosmic Microwave ! Earlier in time, all the matter must have been Background (ch 14) squeezed more tightly together ! If crushed together at high enough density, the galaxies, stars, etc could not exist as we see them now -- everything must have been different! !The Hot Big Bang This week: read Chapter 12/14 in textbook 4/15/14 1 4/15/14 3 Let’s think about the early Universe… Let’s think about the early Universe… ! From Hubble’s observations, we know the Universe is ! From Hubble’s observations, we know the Universe is expanding expanding ! This can be understood theoretically in terms of solutions of ! This can be understood theoretically in terms of solutions of GR equations GR equations ! Earlier in time, all the matter must have been squeezed more tightly together ! If crushed together at high enough density, the galaxies, stars, etc could not exist as we see them now -- everything must have been different! ! What was the Universe like long, long ago? ! What were the original contents? ! What were the early conditions like? ! What physical processes occurred under those conditions? ! How did changes over time result in the contents and structure we see today? 4/15/14 2 4/15/14 4 The Poetic Version ! In a brilliant flash about fourteen billion years ago, time and matter were born in a single instant of creation. -

Section 22-3: Energy, Momentum and Radiation Pressure
Answer to Essential Question 22.2: (a) To find the wavelength, we can combine the equation with the fact that the speed of light in air is 3.00 " 108 m/s. Thus, a frequency of 1 " 1018 Hz corresponds to a wavelength of 3 " 10-10 m, while a frequency of 90.9 MHz corresponds to a wavelength of 3.30 m. (b) Using Equation 22.2, with c = 3.00 " 108 m/s, gives an amplitude of . 22-3 Energy, Momentum and Radiation Pressure All waves carry energy, and electromagnetic waves are no exception. We often characterize the energy carried by a wave in terms of its intensity, which is the power per unit area. At a particular point in space that the wave is moving past, the intensity varies as the electric and magnetic fields at the point oscillate. It is generally most useful to focus on the average intensity, which is given by: . (Eq. 22.3: The average intensity in an EM wave) Note that Equations 22.2 and 22.3 can be combined, so the average intensity can be calculated using only the amplitude of the electric field or only the amplitude of the magnetic field. Momentum and radiation pressure As we will discuss later in the book, there is no mass associated with light, or with any EM wave. Despite this, an electromagnetic wave carries momentum. The momentum of an EM wave is the energy carried by the wave divided by the speed of light. If an EM wave is absorbed by an object, or it reflects from an object, the wave will transfer momentum to the object. -

Measurement of the Cosmic Microwave Background Radiation at 19 Ghz
Measurement of the Cosmic Microwave Background Radiation at 19 GHz 1 Introduction Measurements of the Cosmic Microwave Background (CMB) radiation dominate modern experimental cosmology: there is no greater source of information about the early universe, and no other single discovery has had a greater impact on the theories of the formation of the cosmos. Observation of the CMB confirmed the Big Bang model of the origin of our universe and gave us a look into the distant past, long before the formation of the very first stars and galaxies. In this lab, we seek to recreate this founding pillar of modern physics. The experiment consists of a temperature measurement of the CMB, which is actually “light” left over from the Big Bang. A radiometer is used to measure the intensity of the sky signal at 19 GHz from the roof of the physics building. A specially designed horn antenna allows you to observe microwave noise from isolated patches of sky, without interference from the relatively hot (and high noise) ground. The radiometer amplifies the power from the horn by a factor of a billion. You will calibrate the radiometer to reduce systematic effects: a cryogenically cooled reference load is periodically measured to catch changes in the gain of the amplifier circuit over time. 2 Overview 2.1 History The first observation of the CMB occurred at the Crawford Hill NJ location of Bell Labs in 1965. Arno Penzias and Robert Wilson, intending to do research in radio astronomy at 21 cm wavelength using a special horn antenna designed for satellite communications, noticed a background noise signal in all of their radiometric measurements. -

Black Body Radiation
BLACK BODY RADIATION hemispherical Stefan-Boltzmann law This law states that the energy radiated from a black body is proportional to the fourth power of the absolute temperature. Wien Displacement Law FormulaThe Wien's Displacement Law provides the wavelength where the spectral radiance has maximum value. This law states that the black body radiation curve for different temperatures peaks at a wavelength inversely proportional to the temperature. Maximum wavelength = Wien's displacement constant / Temperature The equation is: λmax= b/T Where: λmax: The peak of the wavelength b: Wien's displacement constant. (2.9*10(−3) m K) T: Absolute Temperature in Kelvin. Emissive power The value of the Stefan-Boltzmann constant is approximately 5.67 x 10 -8 watt per meter squared per kelvin to the fourth (W · m -2 · K -4 ). If the radiation emitted normal to the surface and the energy density of radiation is u, then emissive power of the surface E=c u If the radiation is diffuse Emitted uniformly in all directions 1 E= 푐푢 4 Thermal radiation exerts pressure on the surface on which they are Incident. If the intensity of directed beam of radiations incident normally to The surface is I 퐼 Then Pressure P=u= 푐 If the radiation is diffused 1 P= 푢 3 The value of the constant is approximately 1.366 kilowatts per square metre. When the emissivity of non-black surface is constant at all temperatures and throughout the entire range of wavelength, the surface is called Gray Body. PROBLEMS 1. The temperature of a person’s skin is 350 C. -
Properties of Electromagnetic Waves Any Electromagnetic Wave Must Satisfy Four Basic Conditions: 1
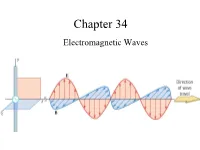
Chapter 34 Electromagnetic Waves The Goal of the Entire Course Maxwell’s Equations: Maxwell’s Equations James Clerk Maxwell •1831 – 1879 •Scottish theoretical physicist •Developed the electromagnetic theory of light •His successful interpretation of the electromagnetic field resulted in the field equations that bear his name. •Also developed and explained – Kinetic theory of gases – Nature of Saturn’s rings – Color vision Start at 12:50 https://www.learner.org/vod/vod_window.html?pid=604 Correcting Ampere’s Law Two surfaces S1 and S2 near the plate of a capacitor are bounded by the same path P. Ampere’s Law states that But it is zero on S2 since there is no conduction current through it. This is a contradiction. Maxwell fixed it by introducing the displacement current: Fig. 34-1, p. 984 Maxwell hypothesized that a changing electric field creates an induced magnetic field. Induced Fields . An increasing solenoid current causes an increasing magnetic field, which induces a circular electric field. An increasing capacitor charge causes an increasing electric field, which induces a circular magnetic field. Slide 34-50 Displacement Current d d(EA)d(q / ε) 1 dq E 0 dt dt dt ε0 dt dq d ε E dt0 dt The displacement current is equal to the conduction current!!! Bsd μ I μ ε I o o o d Maxwell’s Equations The First Unified Field Theory In his unified theory of electromagnetism, Maxwell showed that electromagnetic waves are a natural consequence of the fundamental laws expressed in these four equations: q EABAdd 0 εo dd Edd s BE B s μ I μ ε dto o o dt QuickCheck 34.4 The electric field is increasing. -

Class 12 I : Blackbody Spectra
Class 12 Spectra and the structure of atoms Blackbody spectra and Wien’s law Emission and absorption lines Structure of atoms and the Bohr model I : Blackbody spectra Definition : The spectrum of an object is the distribution of its observed electromagnetic radiation as a function of wavelength or frequency Particularly important example… A blackbody spectrum is that emitted from an idealized dense object in “thermal equilibrium” You don’t have to memorize this! h=6.626x10-34 J/s -23 kB=1.38x10 J/K c=speed of light T=temperature 1 2 Actual spectrum of the Sun compared to a black body 3 Properties of blackbody radiation… The spectrum peaks at Wien’s law The total power emitted per unit surface area of the emitting body is given by finding the area under the blackbody curve… answer is Stephan-Boltzmann Law σ =5.67X10-8 W/m2/K4 (Stephan-Boltzmann constant) II : Emission and absorption line spectra A blackbody spectrum is an example of a continuum spectrum… it is smooth as a function of wavelength Line spectra: An absorption line is a sharp dip in a (continuum) spectrum An emission line is a sharp spike in a spectrum Both phenomena are caused by the interaction of photons with atoms… each atoms imprints a distinct set of lines in a spectrum The precise pattern of emission/absorption lines tells you about the mix of elements as well as temperature and density 4 Solar spectrum… lots of absorption lines 5 Kirchhoffs laws: A solid, liquid or dense gas produces a continuous (blackbody) spectrum A tenuous gas seen against a hot -

Peter Scicluna – Systematic Effects in Dust-Mass Determinations
The other side of the equation Systematic effects in the determination of dust masses Peter Scicluna ASIAA JCMT Users’ Meeting, Nanjing, 13th February 2017 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 1 / 9 Done for LMC (Reibel+ 2012), SMC (Srinivasan+2016) Total dust mass ∼ integrated dust production over Hubble time What about dust destruction? Dust growth in the ISM? Are the masses really correct? The dust budget crisis: locally Measure dust masses in FIR/sub-mm Measure dust production in MIR Gordon et al., 2014 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 2 / 9 What about dust destruction? Dust growth in the ISM? Are the masses really correct? The dust budget crisis: locally Measure dust masses in FIR/sub-mm Measure dust production in MIR Done for LMC (Reibel+ 2012), SMC (Srinivasan+2016) Total dust mass ∼ integrated dust production over Hubble time Gordon et al., 2014 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 2 / 9 Dust growth in the ISM? Are the masses really correct? The dust budget crisis: locally Measure dust masses in FIR/sub-mm Measure dust production in MIR Done for LMC (Reibel+ 2012), SMC (Srinivasan+2016) Total dust mass ∼ integrated dust production over Hubble time What about dust destruction? Gordon et al., 2014 Peter Scicluna JCMT Users’ Meeting, Nanjing, 13th February 2017 2 / 9 How important are supernovae? Are there even enough metals yet? Dust growth in the ISM? Are the masses really correct? The dust budget crisis: at high redshift Rowlands et al., 2014 Measure -

The Human Ear Hearing, Sound Intensity and Loudness Levels
UIUC Physics 406 Acoustical Physics of Music The Human Ear Hearing, Sound Intensity and Loudness Levels We’ve been discussing the generation of sounds, so now we’ll discuss the perception of sounds. Human Senses: The astounding ~ 4 billion year evolution of living organisms on this planet, from the earliest single-cell life form(s) to the present day, with our current abilities to hear / see / smell / taste / feel / etc. – all are the result of the evolutionary forces of nature associated with “survival of the fittest” – i.e. it is evolutionarily{very} beneficial for us to be able to hear/perceive the natural sounds that do exist in the environment – it helps us to locate/find food/keep from becoming food, etc., just as vision/sight enables us to perceive objects in our 3-D environment, the ability to move /locomote through the environment enhances our ability to find food/keep from becoming food; Our sense of balance, via a stereo-pair (!) of semi-circular canals (= inertial guidance system!) helps us respond to 3-D inertial forces (e.g. gravity) and maintain our balance/avoid injury, etc. Our sense of taste & smell warn us of things that are bad to eat and/or breathe… Human Perception of Sound: * The human ear responds to disturbances/temporal variations in pressure. Amazingly sensitive! It has more than 6 orders of magnitude in dynamic range of pressure sensitivity (12 orders of magnitude in sound intensity, I p2) and 3 orders of magnitude in frequency (20 Hz – 20 KHz)! * Existence of 2 ears (stereo!) greatly enhances 3-D localization of sounds, and also the determination of pitch (i.e. -

Blackbody Radiation
Blackbody Radiation PHY293, PHY324 - TWO WEIGHTS PHY294 - ONE WEIGHT RECOMENDED READINGS 1) R. Harris: Modern Physics, Ch.3, pp. 74-75; Ch.9, pp.384-85. Addison Wesley, 2008. 2) Daniel V. Schroeder: Introduction to Thermal Physics, Ch.7. Addison Wesley Longman Publishing, 2000. 3) PASCO Instruction Manual and Experiment Guide for the Blackbody Radiation at https://www.pasco.com/file_downloads/Downloads_Manuals/Black-Body-Light-Source- Basic-Optics-Manual-OS-8542.pdf 4) Marcello Carla: “Stefan–Boltzmann law for the tungsten filament of a light bulb: Revisiting the experiment”. Am. J. Phys. V. 81 (7), 2013. http://studenti.fisica.unifi.it/~carla/varie/Stefan-Boltzmann_law_in_a_light_bulb.pdf INTRODUCTION All material objects emit electromagnetic radiation at a temperature above absolute zero. The radiation represents a conversion of a body's thermal energy into electromagnetic energy, and is therefore called thermal radiation. Conversely all matter absorbs electromagnetic radiation to some degree. An object that absorbs all radiation falling on it at all wavelengths is called a blackbody. It is well known that when an object, such as a lump of metal, is heated, it glows; first a dull red, then as it becomes hotter, a brighter red, then bright orange, then a brilliant white. Although the brightness varies from one material to another, the color (strictly spectral distribution) of the glow is essentially universal for all materials, and depends only on the temperature. In the idealized case, this is known as blackbody, or cavity, radiation. At low temperatures, the wavelengths of thermal radiation are mainly in infrared. As temperature increases, objects begin to glow red. -

Reflection and Refraction of Light
Reflection and refraction When light hits a surface, one or more of three things may happen. The light may be reflected back from the surface, transmitted through the material (in which case it will deviate from its initial direction, a process known as refraction) or absorbed by the material if it is not transparent at the wavelength of the incident light. 1. Reflection and refraction Reflection and refraction are governed by two very simple laws. We consider a light wave travelling through medium 1 and striking medium 2. We define the angle of incidence θ as the angle between the incident ray and the normal to the surface (a vector pointing out perpendicular to the surface). For reflection, the reflected ray lies in the plane of incidence, and θ1’ = θ1 For refraction, the refracted ray lies in the plane of incidence, and n1sinθ1 = n2sinθ2 (this expression is called Snell’s law). In the above equations, ni is a dimensionless constant known as the θ1 θ1’ index of refraction or refractive index of medium i. In addition to reflection determining the angle of refraction, n also determines the speed of the light wave in the medium, v = c/n. Just as θ1 is the angle between refraction θ2 the incident ray and the surface normal outside the medium, θ2 is the angle between the transmitted ray and the surface normal inside the medium (i.e. pointing out from the other side of the surface to the original surface normal) 2. Total internal reflection A consequence of Snell’s law is a phenomenon known as total internal reflection. -

Chapter 12: Physics of Ultrasound
Chapter 12: Physics of Ultrasound Slide set of 54 slides based on the Chapter authored by J.C. Lacefield of the IAEA publication (ISBN 978-92-0-131010-1): Diagnostic Radiology Physics: A Handbook for Teachers and Students Objective: To familiarize students with Physics or Ultrasound, commonly used in diagnostic imaging modality. Slide set prepared by E.Okuno (S. Paulo, Brazil, Institute of Physics of S. Paulo University) IAEA International Atomic Energy Agency Chapter 12. TABLE OF CONTENTS 12.1. Introduction 12.2. Ultrasonic Plane Waves 12.3. Ultrasonic Properties of Biological Tissue 12.4. Ultrasonic Transduction 12.5. Doppler Physics 12.6. Biological Effects of Ultrasound IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 12,2 12.1. INTRODUCTION • Ultrasound is the most commonly used diagnostic imaging modality, accounting for approximately 25% of all imaging examinations performed worldwide nowadays • Ultrasound is an acoustic wave with frequencies greater than the maximum frequency audible to humans, which is 20 kHz IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 12,3 12.1. INTRODUCTION • Diagnostic imaging is generally performed using ultrasound in the frequency range from 2 to 15 MHz • The choice of frequency is dictated by a trade-off between spatial resolution and penetration depth, since higher frequency waves can be focused more tightly but are attenuated more rapidly by tissue The information in an ultrasonic image is influenced by the physical processes underlying propagation, reflection and attenuation of ultrasound waves in tissue IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 12,4 12.1.