Nexgard Technical Alert
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rockpile, Fevrier 96 the Flea
ROCKPILE, FEVRIER 96 THE FLEA INTERVIEW by André Calilhanna Flea, the infamous bassist of the Red Hot Chili Peppers, hardly needs much of an introduction. Having defined the criteria by which modern slap and pop rock bass is measured, he is something of an icon in the halls of rock and roll. W ith a new album, and another new guitarist in Dave Navarro, The Red Hot Chili Peppers have embarked on another leg in their dynamic and often troubled careers. Rockpile's André Calilhanna had the opportunity to talk with Flea about life, the universe and everything just as the Chilis ready themselves for a tour of the United States. With (Dave) Navarro joining the band, things have changed a lot, I'm assuming, in the way you guys were writing. I mean, you've got another writer in the mix right now... FLEA: Yeah, well, he's more of a reactor than he is a writer. He's a different kind of creator. He's more a reactor than someone who'll say, 'here I've got this part...' Yeah, there are certain parts of the album where I guess I understand because it was more in the solos and in parts that are sort of over the core of the music that I thought I could hear his influence, over top something that was more of a Chili Peppers' sound. What was it like working with him? Was it easy ? Was it tough ? I mean, I know you guys had been courting him for a little while. -
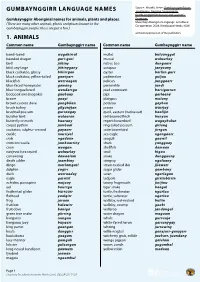
Gumbaynggirr Language Database
Source: Morelli, Steve: Gumbaynggirr Bijaarr GUMBAYNGGIRR LANGUAGE NAMES Jandaygam, Ngaawa Gugaarrigam Gumbaynggirr Dictionary and Learner’s Gumbaynggirr Aboriginal names for animals, plants and places. Grammar. (There are many other animals, plants and places known to the Muurrbay Aboriginal Language & Cultural Co-operative, 2008, Nambucca Heads, NSW. Gumbaynggirr people: these are just a few.) with kind permission of the publishers. 1. ANIMALS Common name Gumbaynggirr name Common name Gumbaynggirr name bandi-bandi wagabirrul mullet buluunggal bearded dragon guri-guri mussel waluurlay bird jiibiny native bee dungaarr bird, any large jiibinygany octopus janyaany black cockatoo, glossy biliirrgan oyster barliin.gurr black cockatoo, yellow-tailed gawiyarr pademelon guljuu blackfish warraagan pelican junggaarr blue-faced honeyeater gawang periwinkle jundi blue-tongue lizard wandarrga pied cormorant barrigurrun boobook owl (mopoke) guubuny pipi garlaany bream gaayi platypus muluny brown cuckoo dove gungbilan potaroo gayban brush turkey gilgunyjan prawn wurrjay brushtail possum gurraagay quoll, eastern (native cat) baalijin butcher bird wulaaran red-browed finch bunyun butterfly or moth baarany regent bowerbird wagaybulun carpet python jumbaal ring-tailed possum giilung cockatoo, sulphur-crested gayaarr satin bowerbird jirrgan cockle murriyal sea eagle ngangaarr crab ngaduun seagull gaawil crimson rosella jambaarriny shark yanggaay crow waagan shellfish damaan cunjevoi (sea squirt) waluurlay snail bigaa currawong dawaalam snake dungguuny death -

Chris Donovan AEA SAG-AFTRA ACTOR/SINGER EYES: GREEN (347) 262-6009 TENOR/BARITONE HEIGHT: 6’3” [email protected] HAIR: LIGHT BROWN WEIGHT: 200LBS
Chris Donovan AEA SAG-AFTRA ACTOR/SINGER EYES: GREEN (347) 262-6009 TENOR/BARITONE HEIGHT: 6’3” [email protected] HAIR: LIGHT BROWN WEIGHT: 200LBS THEATRE THE BEST LITTLE WHOREHOUSE IN TEXAS SENATOR/MAYOR NATIONAL TOUR A MIDNIGHT CLEAR ELIAS TOMPKINS THE RIVERSIDE THEATRE MACBETH BANQUO (U/S MACBETH) EDINBURGH FRINGE FESTIVAL DAMN YANKEES DELL (U/S JOE/APPLEGATE) THE RIVER REP A FLEA IN HER EAR HOTEL GUEST (U/S CHANDEBISE/POCHE) THE RIVER REP ART U/S YVONNE/SERGE/MARC THE RIVER REP BUS STOP U/S BO DECKER/WILL MASTERS THE RIVER REP ANNIE AND BILL FRANK BUTLER FANFARE CHILDREN’S THEATER OFF-OFF BROADWAY THEATRE THE WILL OF LOVE (SHAKESPEARE) BENEDICK THE SHELL THEATER DELILAH YA’IR/DAVID MIDTOWN FESTIVAL THE CHERRY ORCHARD LOPAHIN THE RAW SPACE THE AMERICAN GIRLS REVUE UNCLE GARD/JIGGY NYE AMERICAN GIRLS NYC KING JOHN THE BASTARD THE PRODUCERS’ CLUB II THE MAN WHO HATED SHAKESPEARE BONE CRUSHER THE PRODUCERS’ CLUB TWELFTH NIGHT TOBY BELCH TODO 45 UNIVERSITY THEATRE THE TRESTLE AT POPE LICK CREEK CHAS WEAVER BROOKLYN COLLEGE CLYBOURNE PARK RUSS/DAN BROOKLYN COLLEGE THE RIMERS OF ELDRITCH JUDGE/PREACHER BROOKLYN COLLEGE INTO THE WOODS RAPUNZEL’S PRINCE CIRCLE IN THE SQUARE THE CHILDREN’S HOUR DR. JOSEPH CARDIN CIRCLE IN THE SQUARE SHAKESPEARE’S VILLAINS VARIOUS SHAKESPEARE CHARACTERS WESTERN CONNECTICUT STATE A FLEA IN HER EAR DR. FINACHE WESTERN CONNECTICUT STATE A MIDSUMMER NIGHT’S DREAM THESEUS WESTERN CONNECTICUT STATE THE GLASS MENAGERIE JIM WESTERN CONNECTICUT STATE CITY OF ANGELS STINE WESTERN CONNECTICUT STATE FIDDLER ON THE -

Palatability of Plants to Camels (DBIRD NT)
Technote No. 116 June 2003 Agdex No: 468/62 ISSN No: 0158-2755 The Palatability of Central Australian Plant Species to Camels Dr B. Dorges, Dr J. Heucke, Central Australian Camel Industry Association and R. Dance, Pastoral Division, Alice Springs BACKGROUND About 600,000 camels (Camelus dromedarius) are believed to inhabit the arid centre of Australia, mainly in South Australia, Western Australia and the Northern Territory. Most of these camels are feral. A small camel industry has developed, which harvests selected animals for domestic and export markets, primarily for meat. Camels can eat more than 80% of the common plant species found in Central Australia. Some plant species are actively sought by camels and may need to be protected. METHOD Observations of grazing preferences by camels were made periodically for up to 12 years on five cattle stations in Central Australia. Where camels were accustomed to the presence of humans, it was possible to observe their grazing preferences from a few metres. Radio transmitters were fitted on some camels for easy detection and observation at any time. These evaluations were used to establish a diet preference or palatability index for observed food plants. Table 1. Palatability index for camels Index Interpretation 1 only eaten when nothing else is available 2 rarely eaten 3 common food plant 4 main food plant at times 5 preferred food plant 6 highly preferred food plant 7 could be killed by camel browsing More information can be obtained from the web site of the Central Australian Camel Industry Association http://www.camelsaust.com.au 2 RESULTS Table 2. -

Why the Red Hot Chili Peppers?
Red Hot Chili Peppers Group 3 Kaitlin Gaedtke Lauren Lalumiere Maisey Pierce Why the Red Hot Chili Peppers? The Red Hot Chili Peppers are an innovative rock group formed in the early 80s. They have seemingly always been hugely influential in the style of rock and alternative rock. Still successful in creating music and performing today. Group Biography RHCP were formed from a California high school friendship and a mutual appreciation for punk rock and funk. Started out as “Tony Flow & the Miraculously Majestic Masters of Mayhem,” this is also where their performing nude “trademark” started They began a recording contract with EMI and gained popularity when their videos were featured on MTV Group Biography (Cont.) Several members fought drug addiction along the way; one (Slovak) even died from a heroin overdose. They’ve had many different members but current members Kiedis and Flea are founders Flea (left in both pictures) and Kiedis (right in both pictures) have been friends since they went to highschool together! Current Members Anthony Kiedis: lead vocals (1983-present) Flea: bass, trumpet, piano, backing vocals (1983-present) Chad Smith: drums, percussion (1988-present) Josh Klinghoffer: guitar, keyboards, backing vocals (2009-present) Anthony Kiedis: went to highschool with Flea in California, where they acknowledged their mutual admiration of punk rock and funk groups by forming a band together. talent for poetry and was convinced by Flea and Slovak to write music. Even after his friend Slovak’s death due to an overdose, Kiedis still battled a drug addiction. In the 2000’s, he attempted to turn his autobiography called “Scar Tissue” into a TV show, which failed. -

Snow Patrol ‘Chasing Cars’
Rockschool Grade Pieces Snow Patrol ‘Chasing Cars’ Snow Patrol SONG TITLE: CHASING CARS ALBUM: EYES OPEN RELEASED: 2006 LABEL: POLYDOR GENRE: INDIE PERSONNEL: GARY LIGHTBODY (VOX+GTR) NATHAN CONNOLLY (GTR) PAUL WILSON (BASS) JONNY QUINN (DRUMS) TOM SIMPSON (KEYS) UK CHART PEAK: 6 US CHART PEAK: 5 BACKGROUND INFO NOTES ‘Chasing Cars’ is the second single from Snow Although it didn’t achieve a number 1 in the UK or Patrol’s 2006 album Eyes Open. It is a based on a the U.S. ‘Chasing Cars’ still receives massive airplay single three-chord progression, but ‘Chasing Cars’ is and can be heard almost constantly in TV shows. A far from simple. The song starts with a sparse picked moving acoustic version of ‘Chasing Cars’ appears on eighth-note guitar line which is augmented by subtle the soundtrack for the US TV show Grey’s Anatomy. keyboard parts. The arrangement uses changes in dynamics to develop the song. The third chorus sees ‘Chasing Cars’ move up another notch adding RECOMMENDED LISTENING drums and several distorted guitars playing different inversions (where the notes of a chord are arranged Snow Patrol’s songs are masterpieces of in a different order) to create an orchestra-like wall of arrangement and see the guitar adopting a supporting guitars. The end of the song sees the song return to role on their songs rather than the dominant riffs its sparse beginnings with the re-stating of the simple and extended guitar solos you might expect to hear picked guitar part. from a rock, blues or metal band. -

The Flea Theater
THE FLEA THEATER JIM SIMP S ON ARTISTIC DIRECTOR CAROL OS TROW PRODUCING DIRECTOR BETH DEM B ROW MANAGING DIRECTOR PRESENTS THE WORLD PREMIERE OF A LETTER FROM OMDURMAN WRITTEN BY JEFFREY M. JONE S DIRECTED BY PAGE BURKHOL D ER FEATURING HE AT S T B KATE SIN C LAIR FO S TER SET DESIGN JONATHAN COTTLE LIGHTING DESIGN WHITNEY LO C HER COSTUME DESIGN COLIN WHITELY SOUND DESIGN DAN DURKIN PROJECTION DESIGN JULON D RE BROWN STAGE MANAGMENT A LETTER FROM OMDURMAN CAST (IN ORDER OF APPEARANCE ) Dave...............................................................................................................Matt Barbot Charlie..........................................................................................................Wilton Yeung Wyatt................................................................................................................Will Turner Josie........................................................................................................Veracity Butcher Understudies: Eric Folks (Wyatt), Danny Rivera (Charlie & Dave) CREATIVE TEAM Playwright.............................................................................................................Jeffrey M. Jones Director................................................................................................................Page Burkholder Set Design......................................................................................................Kate Sinclair Foster Lighting Design......................................................................................................Jonathan -

Managing Pesticide Poisoning Risk and Understanding the Signs and Symptoms Clyde L
EC2505 Revised June 2018 Managing Pesticide Poisoning Risk and Understanding the Signs and Symptoms Clyde L. Ogg, Extension Educator Jan R. Hygnstrom, Project Manager Cheryl A. Alberts, Project Coordinator Erin C. Bauer, Entomology Lecturer The potential for accidents with pesticides is real. Ac- cidental exposure or overexposure to pesticides can have seri- ous consequences. While most pesticides can be used with relatively little risk when label directions are followed, some are extremely toxic and require special precautions. The Poison Control Centers receive about 90,000 calls each year related to pesticide exposures. Pesticides are re- sponsible for about 3 percent of all accidental exposures to children 5 years and younger and about 4 percent for adults. In addition, pesticides are the cause of about 3 percent of children’s deaths reported to the Poison Control Centers. Routes of Exposure Pesticides can enter the human body three ways: 1) der- mal exposure, by absorption through the skin or eyes; 2) oral exposure, through the mouth; and 3) through inhalation or respiratory exposure, by inhaling into the lungs. Some classify exposure through the eyes as ocular exposure. Dermal exposure results in absorption immediately after Figure 1. Absorption rates of different a pesticide contacts the skin or eyes. Absorption will contin- parts of the body based on the absorption ue as long as the pesticide remains in contact with the skin or of parathion into the forearm over 24 eyes. The rate at which dermal absorption occurs is different hours. for each part of the body (Figure 1). Maiback and Feldman (1974) measured the amount of the pesticide parathion absorbed by different parts of the human body over 24 hours. -

Native Species
Birdlife Australia Gluepot Reserve PLANT SPECIES LIST These are species recorded by various observers. Species in bold have been vouchered. The list is being continually updated NATIVE SPECIES Species name Common name Acacia acanthoclada Harrow Wattle Acacia aneura Mulga Acacia brachybotrya Grey Mulga Acacia colletioides Wait a While Acacia hakeoides Hakea leaved Wattle Acacia halliana Hall’s Wattle Acacia ligulata Sandhill Wattle Acacia nyssophylla Prickly Wattle Acacia oswaldii Boomerang Bush Acacia rigens Needle Wattle Acacia sclerophylla var. sclerophylla Hard Leaved Wattle Acacia wilhelmiana Wilhelm’s Wattle Actinobole uliginosum Flannel Cudweed Alectryon oleifolius ssp. canescens Bullock Bush Amphipogon caricinus Long Grey Beard Grass Amyema miquelii Box Mistletoe Amyema miraculosa ssp. boormanii Fleshy Mistletoe Amyema preissii Wire Leaved Acacia Mistletoe Angianthus tomentosus Hairy Cup Flower Atriplex acutibractea Pointed Salt Bush Atriplex rhagodioides Spade Leaved Salt Bush Atriplex stipitata Bitter Salt Bush Atriplex vesicaria Bladder Salt Bush Austrodanthonia caespitosa Wallaby Grass Austrodanthonia pilosa Wallaby Grass Austrostipa elegantissima Elegant Spear Grass Austrostipa hemipogon Half Beard Spear grass Austrostipa nitida Balcarra Spear grass Austrostipa scabra ssp. falcata Rough Spear Grass Austrostipa scabra ssp. scabra Rough Spear Grass Austrostipa tuckeri Tucker’s Spear grass Baeckea crassifolia Desert Baeckea Baeckea ericaea Mat baeckea Bertya tasmanica ssp vestita Mitchell’s Bertya Beyeria lechenaultii Mallefowl -

Red Hot Chili Peppers to the World Stage
The Californication album signalled the welcome return of the Red Hot Chili Peppers to the world stage. Paul Tingen discusses the recording with the album’s engineer, Jim Scott. he ‘Californication’ of the world is in full swing. Of course, it’s been going on for decades, mainly via The trouble began when their guitarist John Frusciante Tthe influence of the likes of Hollywood and Disney- descended into a journey of drug-induced self-destruc- land. But recently there’s been a major accessory to the tion. Four years later several near miracles have global takeover, in the form of the latest album from the happened. Frusciante is off drugs, has a new set of rejuvenated Red Hot Chili Peppers. It’s their most suc- teeth, some skin grafts on his arms and clearly still cessful album yet, topping the hit parades all over the knows how to play the guitar. He has also joined singer world and turning platinum in Australia, New Zealand, Anthony Kiedis, bassist Flea and drummer Chad Smith Canada, Japan and the USA, and gold in almost all to resurrect the Chili Peppers, and together they have European countries. created an engaging album. Most of the tracks have a Apparently the title came out of a visit by singer high-energy rock vibe, but they’re interspersed with Anthony Kiedis to Borneo. He was surprised to see the many touching ballads, of which Scar Tissue became a local culture saturated with images from the US, and Cal- monster hit. ifornia in particular, whether it was Coca-Cola, Marlboro, Californication’s engineer Jim Scott has an impressive- and Hollywood movies, or T-shirts, posters, CDs and ly long list of credits to his name, including Natalie cassettes from Californian bands (including the Red Hot Merchant, Lucinda Williams, Counting Crows, Tom Petty, Chili Peppers themselves). -

John Frusciante - Space Cadet "Raw" (N°163 -1994)
John Frusciante - Space Cadet "Raw" (n°163 -1994) - John Frusciante was the guitarist for the RED HOT CHILI PEPPERS during their climb to stardom, but walked out on them during their last tour. Why? He had a premonition that he had to do it! With his first solo album due, Frusciante reveals he's living in a different dimension, man! ARTIE NELSON freaks out... A skinny guy with bleached hair and pale skin comes down the stairs. He's wearing a blue shirt, is smoking a filterless Camel, and answers to the name of John Frusciante. Yes, THE JOHN FRUSCIANTE, ex-Red Hot Chili Pepper who's now embarking on a solo career. He sits next to me on an old two-piece shocking velvet couch from which we can take in a panoramic view of Los Angeles. With plenty of Evian water and Fruit Smoothie drinks on the table, the topic of conversation is naturally John's new album, called 'Niandra La Des And Usually Just A T-Shirt'! It's out early next year on Rick Rubin's American Recordings label. "It has the vibe of when I was recording it" Frusciante begins. "Just for myself to trip out. It had the same kind of vibe as Jane's Addiction without drums. It seemed just as heavy. Some people are after as macho thug's presence (with the drums), but as long as the feeling of macho stud is there ..." When did you finish the album? "About a year ago. But it's taken this long to get it out, because it was recorded so casually. -

HOW to MAKE FLEAS… FLEE! the Willamette Valley Provides a Perfect Environment for Everything to Grow, Especially Fleas
503-391-CATS 391-2287 AAHA CERTIFIED 1212 WALLACE RD. N.W. * SALEM, OREGON 97304 503-581-8161 HOSPITAL 503-371-3154 FAX HOW TO MAKE FLEAS… FLEE! The Willamette Valley provides a perfect environment for everything to grow, especially fleas. Since we rarely have freezes of any length, we have a flea population all year long and therefore, the necessity for year round flea control. Fleas are responsible for some serious health problems dogs and cats. Flea allergy dermatitis, for example, is the most commonly treated skin disorder in veterinary medicine. Fleas are responsible for the most common type of tapeworm affecting dogs and cats. Fleas also decrease quality of life for our pets, due to the itching that occurs after a bite. It is important to understand the basic stages of the flea life cycle so that the individual steps involved in control can be easily understood. • The Adult Flea can live 6 to 12 months. Larvae 34% Pupae 8% Adults 1% • The female lays 3 to 30 eggs a day. • If one pet has fleas, it can be assumed that all pets in the house have fleas and need to be treated. Eggs 57% • As the graph indicates, the adult fleas make up only 1% of the population. Because the larger flea population resides in the environment, it is very important to treat the house and yard also or control may not be achieved. PRODUCTS TO USE: Treatment for the Pet: Cheristin ™ is another topical flea treatment for cats only. • Starts killing fleas within 30 minutes after application.