5.7.5 Spermatogenesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Male Reproductive System
MALE REPRODUCTIVE SYSTEM DR RAJARSHI ASH M.B.B.S.(CAL); D.O.(EYE) ; M.D.-PGT(2ND YEAR) DEPARTMENT OF PHYSIOLOGY CALCUTTA NATIONAL MEDICAL COLLEGE PARTS OF MALE REPRODUCTIVE SYSTEM A. Gonads – Two ovoid testes present in scrotal sac, out side the abdominal cavity B. Accessory sex organs - epididymis, vas deferens, seminal vesicles, ejaculatory ducts, prostate gland and bulbo-urethral glands C. External genitalia – penis and scrotum ANATOMY OF MALE INTERNAL GENITALIA AND ACCESSORY SEX ORGANS SEMINIFEROUS TUBULE Two principal cell types in seminiferous tubule Sertoli cell Germ cell INTERACTION BETWEEN SERTOLI CELLS AND SPERM BLOOD- TESTIS BARRIER • Blood – testis barrier protects germ cells in seminiferous tubules from harmful elements in blood. • The blood- testis barrier prevents entry of antigenic substances from the developing germ cells into circulation. • High local concentration of androgen, inositol, glutamic acid, aspartic acid can be maintained in the lumen of seminiferous tubule without difficulty. • Blood- testis barrier maintains higher osmolality of luminal content of seminiferous tubules. FUNCTIONS OF SERTOLI CELLS 1.Germ cell development 2.Phagocytosis 3.Nourishment and growth of spermatids 4.Formation of tubular fluid 5.Support spermiation 6.FSH and testosterone sensitivity 7.Endocrine functions of sertoli cells i)Inhibin ii)Activin iii)Follistatin iv)MIS v)Estrogen 8.Sertoli cell secretes ‘Androgen binding protein’(ABP) and H-Y antigen. 9.Sertoli cell contributes formation of blood testis barrier. LEYDIG CELL • Leydig cells are present near the capillaries in the interstitial space between seminiferous tubules. • They are rich in mitochondria & endoplasmic reticulum. • Leydig cells secrete testosterone,DHEA & Androstenedione. • The activity of leydig cell is different in different phases of life. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Cytology and Kinetics of Spermatogenesis in the Rabbit
CYTOLOGY AND KINETICS OF SPERMATOGENESIS IN THE RABBIT E. E. SWIERSTRA and R. H. FOOTE Department of Animal Husbandry, Cornell University, Ithaca, New York, U.S.A. {Received 21st August 1962) Summary. The cycle of the seminiferous epithelium of the rabbit was divided into eight stages, using as criteria the shape of the spermatid nucleus, the location of the spermatids and spermatozoa in regard to the basement membrane, the presence of meiotic figures and the release of spermatozoa from the lumen. The relative duration (frequency) of Stages 1 to 8 were 27-7, 13-4, 7-3, 11-0, 4-1, 15-7, 12-2 and 8-6%, respectively. Each stem cell (Type A spermatogonium) divided to produce two Type A spermatogonia. One of these was the starting cell for the next genera¬ tion, while the other gave rise to two intermediate-type spermatogonia. Three more spermatogonial divisions followed, producing sixteen primary spermatocytes from one Type A spermatogonium, as is characteristic for the bull and the ram, but unlike the rat, mouse and hamster. It was estimated that only 3-1 spermatids were generated from one primary spermatocyte, suggesting that in the rabbit there is considerable degeneration of spermatogenic cells during the two maturation divisions. INTRODUCTION Since the end of the last century, it has been known that well-defined cellular associations succeed one another in time in any one area of the semini¬ ferous tubules, and that along the tubules a more or less regular pattern of cell populations exists (Brown, 1885; Benda, 1887; von Ebner, 1888). This succession of cellular associations at any one location in the seminiferous tubules led to the concept of the cycle of the seminiferous epithelium defined by Leblond & Clermont (1952b) as that "series of changes occurring in a given area of the seminiferous epithelium between two successive appearances of the same cellular association". -

Male Reproductive Organs Testes (Paired Gonads)
Male Reproductive Organs Testes (paired Gonads) Penis Series of passageways . Epididymis . Ductus Deferens . Urethra Accessory Glands . Seminal vesicle . Prostate Functions • Paired Gonads (Testes) – Produce Spermatozoa (male germ cells) & Androgens (male sex hormones) •Penis– Copulatory organ • Series of passageways & ducts – To store the spermatozoa , ready for delivery to male copulatory organ • Male accessory glands – provide fluid vehicle for carrying spermatozoa Coverings Tunica Vaginalis Tunica Albuginea Tunica Vasculosa Outermost Layer . Tunica Albuginea (Dense connective tissue fibrous Memb.) – Consist of closely packed collagen Fibres with a few Elastic Fibres . form septa ,Project from Mediastinum Testis . Divide incompletely into pyramidal lobules with apex towards Mediatinum . Each Testis Approx-200 lobule . Each lobule has Approx1-4 seminiferous Tubules . Form loop to end in Straight tubule (20-30) • Straight tubules end up unite to form network (Rete testis) which gives off 15-20 efferent ductules • Space between tubules filled up by Loose connective tissue (collagen fibres & fibroblasts,macrophases , mast cells), blood vessels, Lymphatics & Interstitial cells of Leydig Seminiferous Tubules • Fill most of interior of Each Testes • Two types of cells • Germ cells (represent different stages of spermatogenesis) Spermatogonia (Type A & type B) Primary spermatocyte Secondary spermatocyte Spermatids Spermatozoa • Sustantacular cells (Sertoli) Mitosis Spermatogonium 44+X 44+X Type A +Y +Y Spermatogonium 44+X+ Y Type B Enlarge/Mitosis -

Triggers of Spermatogenesis Moses Bibi Moses D
Triggers of Spermatogenesis Moses Bibi Moses D. Bibi will graduate in January 2020 with a Bachelor of Science Honors degree in Biology, is accepted in the Touro Medical Honors Pathway with New York Medical College and will attend medical school beginning fall 2020. Abstract Of the 7% of men affected with infertility, about 54% suffer from pre-testicular and/or testicular factor induced azoospermia/ oligospermia .This agenesis of spermatozoa has been the subject of much andrology research over the past 50 years, with a par- ticular focus in the triggers of spermatogenesis .While much of their work is limited to murine populations, researchers have put a lot of emphasis on the spermatogonial stem cell (SSC) niche as the source of the trigger(s) . By following physiological patterns exhibited in the seminiferous epithelium, researchers have been able to detect distinct morphological stages that correlate with spermatogonial germ-line action . Different niche cells appear to release different concentrations of active compounds, androgens, and receptors during different stages. Specifically, in the steps leading up to and during SSC differentiation, Sertoli cells and germ line cells release retinoic acid and retinoic acid receptors . Retinoic acid appears to trigger SSCs in vitro as well .Testosterone, re- leased by Leydig cells and potentially testicular macrophages, appears to play an essential role in a spermiation-SSC differentiation axis, as well as a role in GDNF production by peritubular myoid cells, both required for proper maintenance of SSC populations and commitment to meiosis .With new and promising research being done on the whole of the SSC niche, as opposed to just Sertoli cells, scientists are closer than ever to uncovering the secrets of male fertility . -
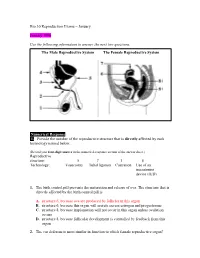
Numerical Response 1. Provide the Number of the Reproductive Structure That Is Directly Affected by Each Technology Named Below
Bio 30 Reproduction Exams – January January 1996 Use the following information to answer the next two questions. The Male Reproductive System The Female Reproductive System Numerical Response 1. Provide the number of the reproductive structure that is directly affected by each technology named below. (Record your four-digit answer in the numerical-response section of the answer sheet.) Reproductive structure: ______5__ ____7____ __1____ ___8_____ Technology: Vasectomy Tubal ligation Castration Use of an intrauterine device (IUD) 1. The birth control pill prevents the maturation and release of ova. The structure that is directly affected by the birth control pill is A. structure 6, because ova are produced by follicles in this organ B. structure 6, because this organ will secrete excess estrogen and progesterone C. structure 8, because implantation will not occur in this organ unless ovulation occurs D. structure 8, because follicular development is controlled by feedback from this organ 2. The vas deferens is most similar in function to which female reproductive organ? A. Ovary B. Uterus C. Vagina D. Fallopian tube Use the following information to answer the next question. Possible Effects of Testosterone 1 Inhibits skeletal muscle development 2 Enhances skeletal muscle development 3 Inhibits development of body hair 4 Promotes development of body hair 5 Inhibits gametogenesis 6 Stimulates gametogenesis 7 Enhances growth of the larynx 8 Inhibits growth of the larynx Numerical Response 2. Select all the correct effects of normal levels of testosterone in an adolescent male. (Record your answer in lowest-to-highest numerical order in the numerical-response section of the answer sheet.) Answer: __2467_____ Use the following information to answer the next two questions. -
![Formation of Gametes [PDF]](https://docslib.b-cdn.net/cover/0353/formation-of-gametes-pdf-1980353.webp)
Formation of Gametes [PDF]
Formation of gametes Dr. Navneet Kumar Professor Anatomy KGMU Lko Dr Navneet Kumar Professor Anatomy KGMU Lko Formation of gametes • Types of gametes – Male gamete = Sperm -Female gamete = Ovum • Process of formation Sperms = Spermatogenesis Ovum = Oogenesis Dr Navneet Kumar Professor Anatomy KGMU Lko Dr Navneet Kumar Professor Anatomy KGMU Lko Spermatogenesis • Site of spermatogenesis- testis • Process (Spermatogenesis) • -Spermatocytosis • -Spermatidogenesis • -Spermeiogesis • Maturation • Structure of Sperm Dr Navneet Kumar Professor Anatomy KGMU Lko Spermatogenesis Site of spermatogenesis - testis • Structure of testis • coverings • -Tunica vaginalis • -Tunica albugenia • -Tunica vasculosa • Cells • Spermatogenic cells • Supporting cells • Interstitial cells • Dr Navneet Kumar Professor Anatomy KGMU Lko Dr. Navneet Kumar Professor Anatomy KGMU Lko Dr Navneet Kumar Professor Anatomy KGMU Lko Dr Navneet Kumar Professor Anatomy KGMU Lko Dr Navneet Kumar Professor Anatomy KGMU Lko Dr Navneet Kumar Professor Anatomy KGMU Lko Supportive cells -Sertoli cells -Provide nutrition Interstitial cells Leyding cells -Secret male hormone at puberty Dr Navneet Kumar Professor Anatomy KGMU Lko Spermatogenesis- process 1.Spermatocytosis • -Primodial or gem cells • -Spermatogonia –type A • -type B • -Primary spermatocyte • -Secondary spermatocyte 2.Spermatidogenesis • -Spermatids 3.Spermeiogenesis Dr Navneet Kumar Professor Anatomy KGMU Lko 1.Spermatocytosis Spermatocytosis- - Initiate at puberty - the primitive germ cells of seminiferous tubules grow and get enlarged then divide in different forms of cells (a)Spermatogonia(44,XY) The primary germ cells undergo division and produce a number of cells termed spermatogonia, Dark- type A cells light -type A cell and type B cells Type B spermatogonia cells- grow as primary spermatocyte . Dr Navneet Kumar Professor Anatomy KGMU Lko 1.Spermatocytosis……. (b) Primary spermatocyte (44,XY) • Type B Spermatogonium cells grow further and from primary spermatocyte. -

Spermatogonial Stem Cells in Fish: Characterization, Isolation, Enrichment, and Recent Advances of in Vitro Culture Systems
biomolecules Review Spermatogonial Stem Cells in Fish: Characterization, Isolation, Enrichment, and Recent Advances of In Vitro Culture Systems Xuan Xie 1,* , Rafael Nóbrega 2 and Martin Pšeniˇcka 1 1 Faculty of Fisheries and Protection of Waters, South Bohemian Research Center of Aquaculture and Biodiversity of Hydrocenoses, University of South Bohemia in Ceske Budejovice, Zátiší 728/II, 389 25 Vodˇnany, Czech Republic; [email protected] 2 Reproductive and Molecular Biology Group, Department of Morphology, Institute of Biosciences, São Paulo State University, Botucatu, SP 18618-970, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +420-606-286-138 Received: 9 March 2020; Accepted: 14 April 2020; Published: 22 April 2020 Abstract: Spermatogenesis is a continuous and dynamic developmental process, in which a single diploid spermatogonial stem cell (SSC) proliferates and differentiates to form a mature spermatozoon. Herein, we summarize the accumulated knowledge of SSCs and their distribution in the testes of teleosts. We also reviewed the primary endocrine and paracrine influence on spermatogonium self-renewal vs. differentiation in fish. To provide insight into techniques and research related to SSCs, we review available protocols and advances in enriching undifferentiated spermatogonia based on their unique physiochemical and biochemical properties, such as size, density, and differential expression of specific surface markers. We summarize in vitro germ cell culture conditions developed to maintain proliferation and survival of spermatogonia in selected fish species. In traditional culture systems, sera and feeder cells were considered to be essential for SSC self-renewal, in contrast to recently developed systems with well-defined media and growth factors to induce either SSC self-renewal or differentiation in long-term cultures. -

Histological Examination of Testicular Cell Development and Apoptosis in the Ostrich Chick
L. WEI, K. M. PENG, H. LIU, H. SONG, Y. WANG, L. TANG Turk. J. Vet. Anim. Sci. 2011; 35(1): 7-14 © TÜBİTAK Research Article doi:10.3906/vet-0806-2 Histological examination of testicular cell development and apoptosis in the ostrich chick Lan WEI1,2, Ke-Mei PENG1,*, Huazhen LIU1, Hui SONG1, Yan WANG1, Lia TANG1 1Department of Anatomy, Histology and Embryology, College of Animal Science and Veterinary Medicine, Huazhong Agricultural University, Wuhan 430070 - CHINA 2College of Animal Science and Technology, Henan University of Science and Technology, Luoyang 471003 - CHINA Received: 06.06.2008 Abstract: Th e aim of this study was to observe the microstructure and ultrastructure of the testis, demonstrate testicular cell development and apoptosis, and elucidate regularity of development and apoptosis in ostrich chick testes. By employing light microscopy 3 obvious development characteristics were detected with ostrich age increasing: fi rst, many primordial germ cells and a few spermatogonia were found while seminiferous tubule integrity was not evident in testes of 1-day-old ostrich chicks nor were primordial germ cells. In the 30-day-old ostrich chicks spermatogonia were completely diff erentiated, but very few primary spermatocytes were observed in testes at 45 days of age. Second, the quantity of mitochondria in spermatogonia and lipid droplet in Leydig cells increased gradually with the increasing age of astrish. Th ird, testicular cell apoptosis was observed and the number of apoptotic testicular cells showed a peak in the testis of 45-day-old ostrich chicks (P < 0.05), as testicular cells were prone to apoptosis at that age. -

26 April 2010 TE Prepublication Page 1 Nomina Generalia General Terms
26 April 2010 TE PrePublication Page 1 Nomina generalia General terms E1.0.0.0.0.0.1 Modus reproductionis Reproductive mode E1.0.0.0.0.0.2 Reproductio sexualis Sexual reproduction E1.0.0.0.0.0.3 Viviparitas Viviparity E1.0.0.0.0.0.4 Heterogamia Heterogamy E1.0.0.0.0.0.5 Endogamia Endogamy E1.0.0.0.0.0.6 Sequentia reproductionis Reproductive sequence E1.0.0.0.0.0.7 Ovulatio Ovulation E1.0.0.0.0.0.8 Erectio Erection E1.0.0.0.0.0.9 Coitus Coitus; Sexual intercourse E1.0.0.0.0.0.10 Ejaculatio1 Ejaculation E1.0.0.0.0.0.11 Emissio Emission E1.0.0.0.0.0.12 Ejaculatio vera Ejaculation proper E1.0.0.0.0.0.13 Semen Semen; Ejaculate E1.0.0.0.0.0.14 Inseminatio Insemination E1.0.0.0.0.0.15 Fertilisatio Fertilization E1.0.0.0.0.0.16 Fecundatio Fecundation; Impregnation E1.0.0.0.0.0.17 Superfecundatio Superfecundation E1.0.0.0.0.0.18 Superimpregnatio Superimpregnation E1.0.0.0.0.0.19 Superfetatio Superfetation E1.0.0.0.0.0.20 Ontogenesis Ontogeny E1.0.0.0.0.0.21 Ontogenesis praenatalis Prenatal ontogeny E1.0.0.0.0.0.22 Tempus praenatale; Tempus gestationis Prenatal period; Gestation period E1.0.0.0.0.0.23 Vita praenatalis Prenatal life E1.0.0.0.0.0.24 Vita intrauterina Intra-uterine life E1.0.0.0.0.0.25 Embryogenesis2 Embryogenesis; Embryogeny E1.0.0.0.0.0.26 Fetogenesis3 Fetogenesis E1.0.0.0.0.0.27 Tempus natale Birth period E1.0.0.0.0.0.28 Ontogenesis postnatalis Postnatal ontogeny E1.0.0.0.0.0.29 Vita postnatalis Postnatal life E1.0.1.0.0.0.1 Mensurae embryonicae et fetales4 Embryonic and fetal measurements E1.0.1.0.0.0.2 Aetas a fecundatione5 Fertilization