Before the Special Tribunal for the Ngaruroro and Clive Rivers Water Conservation Order
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No 82, 1 November 1928, 3119
f,lumb 82. 3119 ",- THE NEW ZEALAND GAZETTE WELLINGTON, THURSDAY, NOVEMBER 1, 1928. Land set apart as an Addition to a Public Domain. the Otokia Creek ; thence towards the north generally by ' said bank, 2650 links ; towards the east generally by the [L.S,] CHARLES FERGUSSON, Governor-General. ocean, 700 links ; towards the south generally by the ocean, A PROCLAMATION. 6100 links ; towards the west by a beach reserve, 60 links ; again towards the north by Beach Street, Township of N pursuance and exercise of the powers conferred by sub Brighton, 2307·2 links ; and towards the west by Bath I section nine of section twelve of the Land Act, 1924, I, Street, 440 links, and the main road, 328 links and 60 links, General Sir Charles Fergusson, Baronet, Governor-General of to the point of commencement, and excluding therefrom a the Dominion of New Zealand, do hereby proclaim and declare closed road intersecting Section 47 and numbered 1318R, a that from and after the date hereof the land comprised in deduction for which has been made from the area. the portions of close~ road describ~d in th~ First Sc~edu le Also, all that area in the Otago Land District containing hereto, being land adiacent to and mtersectmg the Brighton by admeasurement 2 acres, more or less, being Section 60, Domain described in the Second Schedule hereto, shall be Block I, Otokia Survey District, and bounded as follows : rleemed to be added to the said Brighton Domain. Towards the north generally by the Main Road, 87·6 links, 792 links; towards the south by the Ocean, 350 links, and the FIRST SCHEDULE. -

Full Article
NOTORNIS QUARTERLY JOURNAL of the Ornithological Society of New Zealand Volume Sixteen, Number Two, lune, 1969 NOTICE TO CONTRIBUTORS Contributions should be type-written, double- or treble-spaced, with a wide margin, on one side of the paper only. They should be addressed to the Editor, and are accepted o?, condition that sole publication is being offered in the first instance to Notornis." They should be concise, avoid repetition of facts already published, and should take full account of previous literature on the subject matter. The use of an appendix is recommended in certain cases where details and tables are preferably transferred out of the text. Long contributions should be provided with a brief summary at the start. Reprints: Twenty-five off-prints will be supplied free to authors, other than of Short Notes. When additional copies are required, these will be produced as reprints, and the whole number will be charged to the author by the printers. Arrangements for such reprints must be made directly between the author and the printers, Te Rau Press Ltd., P.O. Box 195, Gisborne, prior to publication. Tables: Lengthy and/or intricate tables will usually be reproduced photographically, so that every care should be taken that copy is correct in the first instance. The necessity to produce a second photographic plate could delay publication, and the author may be called upon to meet the additional cost. nlastrutions: Diagrams, etc., should be in Indian ink, preferably on tracing cloth, and the lines and lettering must be sufficiently bold to allow of reduction. Photographs must be suitable in shape to allow of reduction to 7" x 4", or 4" x 3f". -

The Whitestone River by Jr Mills
THE WHITESTONE RIVER BY J.R. MILLS Mills, John (1989) The Whitestone River -- Mills, John (1989) The Whitestone river , . ' . ' . .. _ ' . THE WHITESTONE RIVER John R Mills ---00000--- October 1989 Cover Photo Whitestone River looking upstream towards State Highway 94 bridge and Livingstone Mountain in the background. I. CONTENTS Page number Introduction III Objective ill List of photographs and maps IV Chapter 1 River Description and Location 1.1 Topography 1 1.2 Climate 1 1.3 Vegetation 3 1.4 Soils 3 1.5 Erosion 3 1.6 Water 4 Chapter 2 A Recent History and Factors that have Contributed to the River's Change 6 Chapter 3 Present use and Policy 3.1 Gravel Extraction 8 3.2 Water Rights 8 3.3 Angling 8 3.3a Fishery Requirements 9 3.4 Picnicking 9 3.5 Water Fowl Hunting 9 Chapter 4 Potential Uses 4.1 Grazing 10 4.2 Hay Cutting 10 4.3 Tree Planting 10 Chapter 5 The Public Debate 12 Chapter 6 Man's Interaction with Nature In terms of land development, berm management and their effects on the Whitestone River. 6.1 Scope of Land Development 29 6.2 Berm Boundaries 31 6.3 River Meanders 36 6.4 Protective Planting 39 6.5 Rock and Groyne Works 39 II. Chapter 7 Submissions from Interested Parties 7.1 Southland Catchment Board 42 7.2 Southland Acclimatisation Society 46 - Whitestone River Management and its Trout Fisheries 46 - Submission Appendix Whitestone River Comparison Fisheries Habitat 51 7.3 Farmers Adjoining the River 56 Chapter 8 Options for Future Ownership and Management of the River 57 Chapter 9 Recommendations and Conclusions 9.1a Financial Restraints 59 9.1 b Berm Boundary Constraints 59 9.2 Management Practices 59 9.3 Independent Study 60 9.4 Consultation 60 9.5 Rating 61 9.6 Finally 61 Chapter 10 Recommendations 62 Chapter 11 Acknowledgements 63 ---00000--- III. -
Short Walks in the Invercargill Area Invercargill the in Walks Short Conditions of Use of Conditions
W: E: www.icc.govt.nz [email protected] F: P: +64 3 217 5358 217 3 +64 9070 219 3 +64 Queens Park, Invercargill, New Zealand New Invercargill, Park, Queens Makarewa Office Parks Council City Invercargill For further information contact: information further For Lorneville Lorneville - Dacre Rd North Rd contents of this brochure. All material is subject to copyright. copyright. to subject is material All brochure. this of contents Web: www.es.govt.nz Web: for loss, cost or damage whatsoever arising out of or connected with the the with connected or of out arising whatsoever damage or cost loss, for 8 Email: [email protected] Email: responsibility for any error or omission and disclaim liability to any entity entity any to liability disclaim and omission or error any for responsibility West Plains Rd 9 McIvor Rd 5115 211 03 Ph: the agencies involved in the management of these walking tracks accept no no accept tracks walking these of management the in involved agencies the Waikiwi 9840 Invercargill While all due care has been taken to ensure the accuracy of this publication, publication, this of accuracy the ensure to taken been has care due all While Waihopai Bainfield Rd 90116 Bag Private Disclaimer Grasmere Southland Environment 7 10 Rosedale Waverley www.doc.govt.nz Web: Web: www.southerndhb.govt.nz Web: Bay Rd Herbert St Findlay Rd [email protected] Email: Email: [email protected] Email: Avenal Windsor Ph: 03 211 2400 211 03 Ph: Ph: 03 211 0900 211 03 Ph: Queens Dr Glengarry Tay St Invercargill 9840 Invercargill -

App Pendi Ix 5
Appendix 5 Round the Mountain Cycle Trail – Oreti Valley and Mararoa Valley Route Options – Terrestrial Ecology Report Simon Beale, MWH New Zealand Ltd – September 2014 Tech Time s Round the Mountain Cycle Trail Oreti Valley and Mararoa Valley Route Options Terrestrial Ecology Report Prepared for Southland District Council SEPTEMBER 2014 Southland District Council Round the Mountain Cycle Trail Terrestrial Ecology Report This document has been prepared for the benefit of Southland District Council. No liability is accepted by this company or any employee or sub-consultant of this company with respect to its use by any other person. This disclaimer shall apply notwithstanding that the report may be made available to other persons for an application for permission or approval to fulfil a legal requirement. QUALITY STATEMENT PROJECT MANAGER PROJECT TECHNICAL LEAD Claire Perkins Simon Beale PREPARED BY Simon Beale ………………………………............... 09/09/2014 CHECKED BY ………………………………............... 09/09/2014 Grant Kluyts REVIEWED BY Kristy Hall …………… …………. 09/09/2014 APPROVED FOR ISSUE BY Claire Perkins …………… 09/09/2014 QUEENSTOWN 134a Gorge Rd, Queenstown 9300 PO Box 649, Queenstown 9348 TEL +64 3 450 0890, FAX +64 3 450 0891 REVISION SCHEDULE Rev Signature or Typed Name (documentation on file). Date Description No Prepared by Checked by Reviewed by Approved by Status: Final September 2014 Project number: 80506020 Our ref: Terrestrial Ecology Report Southland District Council Round the Mountain Cycle Trail Terrestrial Ecology Report Southland District -

Anglers' Notice for Fish and Game Region Conservation
ANGLERS’ NOTICE FOR FISH AND GAME REGION CONSERVATION ACT 1987 FRESHWATER FISHERIES REGULATIONS 1983 Pursuant to section 26R(3) of the Conservation Act 1987, the Minister of Conservation approves the following Anglers’ Notice, subject to the First and Second Schedules of this Notice, for the following Fish and Game Region: Southland NOTICE This Notice shall come into force on the 1st day of October 2017. 1. APPLICATION OF THIS NOTICE 1.1 This Anglers’ Notice sets out the conditions under which a current licence holder may fish for sports fish in the area to which the notice relates, being conditions relating to— a.) the size and limit bag for any species of sports fish: b.) any open or closed season in any specified waters in the area, and the sports fish in respect of which they are open or closed: c.) any requirements, restrictions, or prohibitions on fishing tackle, methods, or the use of any gear, equipment, or device: d.) the hours of fishing: e.) the handling, treatment, or disposal of any sports fish. 1.2 This Anglers’ Notice applies to sports fish which include species of trout, salmon and also perch and tench (and rudd in Auckland /Waikato Region only). 1.3 Perch and tench (and rudd in Auckland /Waikato Region only) are also classed as coarse fish in this Notice. 1.4 Within coarse fishing waters (as defined in this Notice) special provisions enable the use of coarse fishing methods that would otherwise be prohibited. 1.5 Outside of coarse fishing waters a current licence holder may fish for coarse fish wherever sports fishing is permitted, subject to the general provisions in this Notice that apply for that region. -

Case Book for Opening Submissions for the Applicants Table Of
Case book for Opening Submissions for the Applicants Table of contents 1. Report of the Special Tribunal on an application to amend the Water Conservation (Buller River) Order, 20 December 2007 2. Rangitata South Irrigation Ltd v New Zealand and Central South Island Fish and Game Council Dec No. C 109/2004 3. Report of the Special Tribunal on a Water Conservation Order Application for the Oreti River, November 2007 4. Interim Decision on Application to Vary the Buller Water Conservation Order, 11 July 2007 5. Re Talley as Trustees for Majac Trust EnvC C006/06 6. Re an Inquiry into the Draft National Water Conservation (Kawarau) Order C33/1996 7. NZ Paper Mills Ltd v Otago Acclimatisation Soc [1995] NZRMA 155 8. Re Whitewater New Zealand Inc [2013] NZEnvC 131 9. Re an Inquiry into the Draft National Water Conservation (Mohaka River) Order W20/92 (PT) 10. Ashburton Acclimatisation Society v Federated Farmers [1988] 1 NZLR 78;12 NZTPA 289 CA 11. Report and Recommendation of the Inquiry into the National Water Conservation Order for the Buller River; PT Wellington, C32/96 Decision No. C I o /2004 4 IN THE MATTER of the Resource Management Act 1991 AND IN THE MATTER of an application for a water conservation order pursuant to section 201 of the Act BETWEEN RANGITATA SOUTH IRRIGATION LIMITED @MA 80’7102) TIMARU DISTRICT COUNCIL (RMA 80X/02) TRUSTPOWER LIMITED @MA 809/02) RANGITATA DIVERSION RACE MANAGEMENT LIMITED @MA 810102) FEDERATED FARMERS OF NEW ZEALAND INCORPORATED (RMA 812102) CANTERBURY REGIONAL COUNCIL (RMA 815102) Submitters AND NEW ZEALAND AND CENTRAL SOUTH ISLAND FISH AND GAME COUNCIL Applicant BEFORE THE ENVIRONMENT COURT Environment Judge J R Jackson (presiding) Environment Commissioner C E Manning Deputy Environment Commissioner R Grigg 2 HEARING at Christchurch on 13-17,20-24 October, 3-7 November 2003; at Timaru on 10. -

Identifying Freshwater Ecosystems with Nationally Important Natural Heritage Values: Development of a Biogeographic Framework
Identifying freshwater ecosystems with nationally important natural heritage values: development of a biogeographic framework J.R. Leathwick, K. Collier and L. Chadderton SCIENCE FOR CONSERVATION 274 Published by Science & Technical Publishing Department of Conservation PO Box 10420, The Terrace Wellington 6143, New Zealand Cover: Ohinemuri River in Karangahake Gorge, Coromandel. Photo: John Leathwick. Science for Conservation is a scientific monograph series presenting research funded by New Zealand Department of Conservation (DOC). Manuscripts are internally and externally peer-reviewed; resulting publications are considered part of the formal international scientific literature. Individual copies are printed, and are also available from the departmental website in pdf form. Titles are listed in our catalogue on the website, refer www.doc.govt.nz under Publications, then Science & technical. © Copyright May 2007, New Zealand Department of Conservation ISSN 1173–2946 ISBN 978–0–478–14207–5 (hardcopy) ISBN 978–0–478–14208–2 (web PDF) This report was prepared for publication by Science & Technical Publishing; editing by Sue Hallas and layout by Lynette Clelland. Publication was approved by the Chief Scientist (Research, Development & Improvement Division), Department of Conservation, Wellington, New Zealand. In the interest of forest conservation, we support paperless electronic publishing. When printing, recycled paper is used wherever possible. ContEnts Abstract 5 1. Introduction 6 1.1 Water-bodies of national importance 6 1.2 Development -
South Island Fishing Regulations for 2020
Fish & Game 1 2 3 4 5 6 Check www.fishandgame.org.nz for details of regional boundaries Code of Conduct ....................................................................4 National Sports Fishing Regulations ...................................... 5 First Schedule ......................................................................... 7 1. Nelson/Marlborough .......................................................... 11 2. West Coast ........................................................................16 3. North Canterbury ............................................................. 23 4. Central South Island ......................................................... 33 5. Otago ................................................................................44 6. Southland .........................................................................54 The regulations printed in this guide booklet are subject to the Minister of Conservation’s approval. A copy of the published Anglers’ Notice in the New Zealand Gazette is available on www.fishandgame.org.nz Cover Photo: Jaymie Challis 3 Regulations CODE OF CONDUCT Please consider the rights of others and observe the anglers’ code of conduct • Always ask permission from the land occupier before crossing private property unless a Fish & Game access sign is present. • Do not park vehicles so that they obstruct gateways or cause a hazard on the road or access way. • Always use gates, stiles or other recognised access points and avoid damage to fences. • Leave everything as you found it. If a gate is open or closed leave it that way. • A farm is the owner’s livelihood and if they say no dogs, then please respect this. • When driving on riverbeds keep to marked tracks or park on the bank and walk to your fishing spot. • Never push in on a pool occupied by another angler. If you are in any doubt have a chat and work out who goes where. • However, if agreed to share the pool then always enter behind any angler already there. • Move upstream or downstream with every few casts (unless you are alone). -
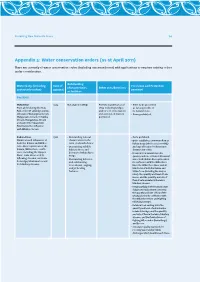
Protecting New Zealand's Rivers Appendix 5
Protecting New Zealand’s Rivers 60 Appendix 5: Water conservation orders (as at April 2011) There are currently 16 water conservation orders (including one amendment) with applications to vary two existing orders under consideration. Outstanding Water body (including %BUFPG Provisions and Protection characteristics Other considerations protected reaches) gazettal provided or features Pre RMA Motu River 1984 Not stated in WCO. Permits maintenance of - River to be preserved From and including the Motu 4)JODMVEJOHCSJEHFT as far as possible in 'BMMTUPUIF4)CSJEHFBOEЯWF and for soil conservation its natural state. tributaries: Waitangirua Stream, and associated matters - Dams prohibited. Mangaotane Stream, Te Kahika permitted Stream, Mangatutara Stream and part of the Takaputahi River below its confluence with Whitikau Stream. Rakaia River 1988 - Outstanding natural - Dams prohibited. Mainstem and tributaries of characteristic in the - Order establishes a minimum flow at both the Rakaia and Wilber- form of a braided river. Rakaia Gorge (which varies monthly) force Rivers upstream of the - Outstanding wildlife and caps allocation for abstraction Rakaia /Wilberforce conflu- habitat above and downstream of this. FODF JODMVEJOHUIF)BSQFS below the Rakaia River - Retain in their natural state the 3JWFS -BLF)FSPOBOEJUT Gorge. quantity and rate of flow of all natural inflowing streams, and Lake - Outstanding fisheries, water in the Rakaia River upstream of Coleridge/Whakamatua and and outstanding its confluence with the Wilberforce its tributary streams. recreational, angling, River, the Wilberforce River, and all and jet boating tributaries of both the Rakaia and features. 8JMCFSGPSDF JODMVEJOHUIF)BSQFS River), the quantity and level of Lake )FSPO BOEUIFRVBOUJUZBOESBUFPG flow of natural water in the lake’s tributary streams. -

Of Hydrological Resea Rch in Progress in N Ew Zealand 19 71
--1 tt -*pr¿-.à-+g Hydrological Research Miscellaneous Publication No 10 A Catalogue of Hydrological Resea rch in Progress in N ew Zealand 19 71 Miscellaneous hydrological publication no. 10 (1972) New Zealand Ministry of !'lorks,'Water and Soi I Division Miscålianeous' ' Hydrological Publications: No. 1: Streamf low correlation in the Vlanganui basin. C. A, A. Smyfh, k re68. # Þ No. 2: Measuring stream discharge using the dye:dilution method (chemical gauging). Hans M. Kel ler, 1969. No. 3z A study of stream behaviour in the Cante¡bury foothills, New Zealand. Hans. M. Kel ler, 1970. No.4:' Hydrological regions of New Zealand. C. Toebes and B. R. Palmer, 1969. No. 5: Field testing of the IAEA portable radioisotope sediment concenlration gauge. W. B. Morrissey, 1970. No.6: The relationship between summer low flows and geology ín Northland, New Zealand. J. R. Waugh, 1970. No. 7z Representative basins of New Zealand, 1970; n.a., 1970. ! No. 8: A catalogue of hydrological research in progress in New Zealand, f ¡ ta 1969; tr.â.r 1970. No. 9: A catalogue of hydrological research in progress. in New Zealand, 1970 n.a., 1971. p i t i I t Miscellaneous hydrological publication no. 10 (1972) MINISTRY 0F l^loRKS - IIATER AND SoIL DMSI0N MISCELLANEOUS HYDROLOGICAL PUBLICATION NO. 10 A CATALOGUE OF HYDROLOGICAL RESEARCH IN PROGRESS IN NEl,l ZEALAND l97L ldellington, New Zealand ' 1972 Miscellaneous hydrological publication no. 10 (1972) This publ icatîon is Produced as a New Zealand conlribution to the I nternational Hydrological Decade, 1965-75, estab I i shed bY UNESCO to furlher infernational co-operation and studY in the science of hYdrology. -

Headwater Trout Fisheries Ln New Zealand
Headwater trout fisheries ln New Zealand D.J. Jellyman E" Graynoth New Zealand Freshwater Research Report No. 12 rssN 1171-9E42 New Zealmtd, Freshwater Research Report No. 12 Headwater trout fïsheries in New Zealand by D.J. Jellyman E. Graynoth NI\ryA Freshwater Christchurch January 1994 NEW ZEALAND FRBSHWATER RESEARCH REPORTS This report is one of a series issued by NItilA Freshwater, a division of the National Institute of Water and Atmospheric Research Ltd. A current list of publications in the series with their prices is available from NIWA Freshwater. Organisations may apply to be put on the mailing list to receive all reports as they are published. An invoice will be sent for each new publication. For all enquiries and orders, contact: The Publications Officer NIWA Freshwater PO Box 8602 Riccarton, Christchurch New Zealand ISBN 0-47848326-2 Edited by: C.K. Holmes Preparation of this report was funded by the New Zealand Fish and Game Councils NIWA (the National Institute of Water and Atmospheric Research Ltd) specialises in meeting information needs for the sustainable development of water and atmospheric resources. It was established on I July 1992. NIWA Freshwater consists of the former Freshwater Fisheries Centre, MAF Fisheries, Christchurch, and parts of the former Marine and Freshwater Division, Department of Scientific and Industrial Research (Hydrology Centre, Christchurch and Taupo Research hboratory). Ttte New Zealand Freshwater Research Report series continues the New Zealand Freshwater Fßheries Report series (formerly the New Zealand. Ministry of Agriculture and Fisheries, Fisheries Environmental Repon series), and Publications of the Hydrology Centre, Chrßtchurch. CONTENTS Page SUMMARY 1.